Se is an essential trace element for human health. Current average Se intake in most countries is sufficient to prevent overt Se-deficiency diseases but may be suboptimal for protection against a number of adverse health conditions(Reference Rayman1). Studies have reported that most of the Australian population have adequate Se intake as determined by dietary intake surveys and plasma Se surveys(Reference Thomson2, Reference Reilly3). Plasma Se levels of 95–100 μg/l are considered to reflect nutritional adequacy. However, since the average plasma Se levels in healthy South Australians were reported as 103 μg/l(Reference Lyons, Judson and Stangoulis4), about one half of the population does not reach desirable Se status. Some groups, in particular, are susceptible to Se deficiency, such as cancer patients, the elderly and smokers. Therefore, a substantial part of the Australian population may have a suboptimal Se status and be potentially responsive to dietary Se supplementation with respect to certain chronic degenerative diseases(Reference Combs5).
There is evidence that low Se status is associated with an increased risk of colorectal cancer (CRC)(Reference Clark, Combs and Turnbull6), whereas a higher Se intake may lower CRC mortality(Reference Russo, Murray and Wurzelmann7, Reference Whanger8), and a higher Se intake is usually associated with a reduced risk of colonic adenoma recurrence(Reference Russo, Murray and Wurzelmann7, Reference Jacobs, Jiang and Alberts9, Reference Connelly-Frost, Poole and Satia10). Thus, Se supplementation may benefit health, including cancer prevention, beyond correction of Se deficiency. However, increasing Se intake is complicated because a number of recent clinical trials have indicated that Se supplementation may have adverse effects on human health(Reference Stranges, Marshall and Trevisan11, Reference Bjelakovic, Nikolova and Gluud12), including a potentially increased risk for type 2 diabetes(Reference Stranges, Marshall and Natarajan13). A recent large Se and Vitamin E Cancer Prevention Trial has also failed to show that Se affects prostate cancer risk(Reference Lippman, Klein and Goodman14), but the trials did not take account of the initial Se status, nor the chemical form of the Se supplement. While synthetic or naturally occurring forms of Se have shown protection against CRC(Reference Rayman, Infante and Sargent15), the question of which is the most effective, bioavailable and functional Se form still needs to be established. Improving Se status through Se from food sources is generally considered preferable to supplementation by pharmaceutical Se products, and interest in foods containing high amounts of Se is increasing(Reference Rayman1, Reference Finley, Davis and Feng16).
Se-enriched milk proteins (dairy-Se) have recently been developed as a novel dairy food by Tatura Milk Industries to provide a significant daily intake of Se for adults(Reference Ortman and Pehrson17, Reference Heard, Stockdale and Walker18); it contains a high concentration of Se (5 parts per million) compared with 0·34 parts per million in normal milk proteins. We have shown in an animal CRC model that dairy-Se was significantly more effective at reducing CRC incidence than Se supplied as Se-rich yeast (yeast-Se)(Reference Hu, McIntosh and Le Leu19). Its protection was associated with significant improvements in relevant biomarkers of CRC risk, namely aberrant crypt foci, K-ras mutations(Reference Hu, McIntosh and Le Leu19) and regulation of colonic epithelial expression of several selenoprotein genes(Reference Hu, McIntosh and Le Leu20).
Se exerts its wide range of biological roles through twenty-five selenoproteins in humans(Reference Gromadzinska, Reszka and Bruzelius21). Some are particularly relevant to anticancer function and have received considerable attention for their important roles in chemoprevention(Reference Rayman22), including selenoprotein P (SeP), cytosolic glutathione peroxidase-1 (GPx-1), gastrointestinal GPx-2 (GPx-2) and thioredoxin reductase-1 (TrxR-1). Selenoprotein levels are dependent on dietary Se intake; currently, dietary reference values for optimal Se intake have mostly relied on blood/plasma Se, or SeP concentrations and GPx activity(Reference Sunde, Paterson and Evenson23, Reference Hurst, Armah and Dainty24), but the changes in these Se status biomarkers do not necessarily reflect expression patterns of selenoproteins in the target tissues, and not all selenoproteins change equally with Se supplementation(Reference Sunde, Paterson and Evenson23, Reference Barnes, Evenson and Raines25). As far as Se supplementation and selenoproteins are concerned, only a small proportion of studies have examined the effect of Se supplementation on selenoprotein expression in the human gastrointestinal tract(Reference Rayman22, Reference Pagmantidis, Meplan and van Schothorst26). Whether selenoproteins specific to different human tissues, for example the colon, are regulated by Se supplementation irrespective of the form is as yet not well determined.
The aim of the present study was to investigate the efficacy of dairy-Se (150 μg Se/d) compared with yeast-Se (150 μg Se/d) on plasma Se levels and, specifically, rectal selenoprotein gene expression in twenty-three healthy volunteers, whose plasma Se levels were in the lower half of the population. A sequential design was followed, where all subjects provided baseline measures, followed by random allocation to either dairy-Se or yeast-Se for 6 weeks, with observations extending throughout the 6-week treatment and 6-week washout period. This design enabled us to assess whether Se supplementation resulted in the regulation of rectal selenoprotein gene expression; we reasoned that if such were not to occur, the capacity to alter the risk for colorectal disease would be low.
Materials and methods
Subjects and recruitment
A group of sixteen male and seven female healthy volunteers aged 52–79 years, considered at risk for CRC by virtue of their age and/or other standard risk factors, were recruited by advertisement. Inclusion criteria were plasma Se concentration at or below 103 μg/l, no evidence of Se supplements or other drug regimens, and with no active bowel disease or previous history of colorectal polyp removal in the last 6 months. This plasma Se level was chosen as it represented the median point of our population and because it has previously been shown that reduced cancer risk is observed only in subjects where plasma Se was at or below 106 μg/l(Reference Rayman27). Exclusion criteria were any allergy or intolerance to milk/dairy products, evidence of any active mucosal bowel disease, e.g. colitis or malabsorption, and evidence of any other active clinical disease precluding participation in the study. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the Flinders Clinical Research Ethics Committee (reference no. 214/067; Flinders Medical Centre, Bedford Park, SA, Australia) and the trial registration number is ACTRN012607000345482. Written informed consent was obtained from all subjects for the biopsies to be used exclusively for the present study.
Selenium supplements
A dairy-Se (Tatura-Bio®Se) was provided by Tatura Milk Industries Limited, Tatura, VIC, Australia. The protein isolate containing this highly bioavailable form of Se was then prepared for human use by Ozscientific Limited (Hoppers Crossing, VIC, Australia) as a water-miscible powder. It contained 70 % protein, 5 % lactose, 4 % moisture, 1·5 % Ca, 1·2 % fat and 5 parts per million Se. A 30 g sachet/d provided 150 μg Se and 21 g milk proteins. Yeast-Se for human use was provided by Alltech Biotech Private Limited (Dandenong South, VIC, Australia) as compressed yeast tablets, with three tablets providing 150 μg Se/d (each tablet contained 50 μg Se, 100 mg lactose and 65 mg microcrystalline cellulose). Both Se products have been analysed for Se compounds: dairy-Se contained 83 % selenomethionine, 5 % selenocysteine and 4 % unknown (R Lobinski, unpublished results); yeast-Se contained 83 % selenomethionine, 5 % selenocysteine and 3 % selenite(Reference Rayman27).
Study protocol
The present study was a 3-month, double-blind and non-cross-over human intervention study, in which subjects were randomly assigned to 6-week dietary supplementation with either dairy-Se or yeast-Se (Fig. 1); all subjects were then observed for a 6-week washout period. Our interest was to assess whether each intervention increased endpoints relative to baseline in these subjects with below average plasma Se and to assess whether any increases were sustained beyond the cessation of supplementation. The dietary approach was pragmatic and allowed participants to readjust the remainder of their diet. Subjects taking dairy-Se were encouraged to substitute this milk protein product in place of two daily dairy serves. Of the twenty-three participants, twelve received dairy-Se (150 μg Se/d) and eleven received yeast-Se (150 μg Se/d) for 6 weeks. Peripheral blood samples were taken every 2 weeks, including 2 or 4 weeks before the study. Rectal biopsies were obtained before Se supplementation (baseline), at the end of Se supplementation and during the washout period. To examine for any potential for intoxication with Se supplements, plasma markers for liver and renal function were assessed. Examination of fasting blood glucose was used to account for any changes in glucose homeostasis. C-reactive protein was also assessed as a measure of acute or chronic inflammation.
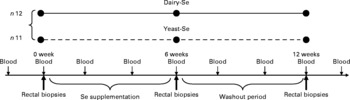
Fig. 1 Overview of blood and tissue sampling and timing of interventions in normal volunteers. Dairy-Se, Se-enriched milk proteins; yeast-Se, Se-rich yeast.
Blood sampling, plasma selenium and glutathione peroxidase activity measurements
Blood samples were collected according to a standardised protocol, placed in 10 ml EDTA-coated tubes, centrifuged for 10 min (3000 g) at 4°C, and plasma samples were stored at − 80°C until analysis. The plasma Se level was measured by atomic absorption spectroscopy using a Perkin Elmer Analyst 800 (PerkinElmer Life and Analytical Sciences, Shelton, CT, USA) with furnace. Plasma GPx activity was determined spectrophotometrically using a glutathione peroxidase assay kit (Cayman Chemical Company, Ann Arbour, MI, USA). GPx activity of 1 unit (U) is defined as 1 μmol NADPH oxidised/min and expressed as U/mg protein.
Processing of biopsies
Rectal biopsies were taken by a gastroenterologist using oversized rectal biopsy forceps; one biopsy was first placed in RNAlater (Applied Biosystems/Ambion, Ambion, Inc., The RNA Company, Austin, TX, USA) solution at − 4°C for 24 h and stored at − 80°C for rectal selenoprotein mRNA expression, and another was formalin fixed and embedded in paraffin for immunohistochemical studies.
Rectal biopsies for RNA isolation and complementary DNA synthesis
Total RNA (30 mg) was extracted from rectal biopsies using a QIAGEN RNeasy Mini Kit (QIAGEN, Hilden, Germany). The concentration and purity of the total RNA was estimated using a NanoDrop® ND-1000 UV–Vis spectrophotometer. First-strand complementary DNA (20 μl) was synthesised from 0·3 μg total RNA for each sample using a QIAGEN QuantiTect Reverse Transcription Kit (QIAGEN).
Quantitative real-time RT-PCR
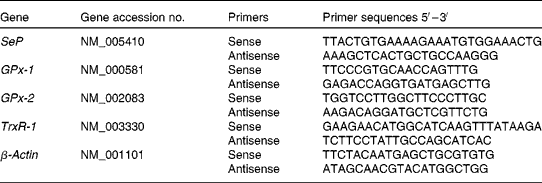
Quantitative real-time RT-PCR was performed in triplicate on a Rotor Gene 3000 Cycler (Corbett, Sydney, NSW, Australia). Oligonucleotide primers were designed using Primer Express software version 1.5 (Applied Biosystems, Inc., Foster City, CA, USA), based on sequences from the GenBank database (Table 1). The PCR was determined in a 20 μl final volume containing 6 μl of diluted 1:30 complementary DNA using the 2 × QuantiTect SYBR Green PCR kit (QIAGEN). The primer concentration for each gene was 10 μm (forward and reverse primers). The cycling protocol was similar as described previously(Reference Hu, McIntosh and Le Leu20). The specificity of PCR was confirmed by melting curve analysis of the amplified PCR products. For each PCR run, a non-template reaction was included as the negative control.
Table 1 Oligonucleotide primers used for real-time RT-PCR
Cycle thresholds were determined using the relative quantification analysis module in the Rotorgene 3000 Series software (Corbett). The amplification efficiency of each primer pair was estimated from a real-time PCR dilution curve generated using serial dilutions of complementary DNA. Quantitative real-time PCR analysis was then performed using Q-Gene software (Integrated DNA Technologies, Inc., Coralville, IA, USA)(Reference Simon28), with the amplification efficiency applied to the relative concentration analyses of both the genes of interest and the housekeeping gene (β-actin). Gene-of-interest expression data were normalised by dividing by the corresponding levels of β-actin for each sample.
Rectal epithelial histopathological analysis
Rectal biopsies were formalin fixed, embedded in paraffin and cut into 5 μm sections for haematoxylin and eosin and immunohistochemistry staining. A total of twenty full-length, non-bifid crypts were selected from each volunteer and counted for the rate of Ki-67-positive cells and crypt height. The tissue was orientated to optimise the longitudinal crypt axis. Ki67 immunohistochemistry was performed using a mouse anti-Ki67 monoclonal primary antibody (M7248; DakoCytomation, Copenhagen, Denmark) and a level 2 Ultra Streptavidin detection system (Signet Laboratories, Inc., Dedham, MA, USA). The proliferation index was calculated as the percentage of Ki67-stained cells per crypt column divided by the total number of cells within the crypt column, reported as a mean proliferation index (%). Cell heights are reported as the mean number of cells per crypt column.
Statistical analysis
All analyses were provided using STATA software (Stata Corporation, College Station, TX, USA) version 10.1. Results are expressed as means with their standard errors. Comparisons of standard blood markers between dairy-Se and yeast-Se at baseline, after Se intervention and during the washout period were performed using the Mann–Whitney test. Within-group comparisons (plasma biomarkers, rectal selenoprotein genes and cell kinetics) between baseline and each time point were performed using a random intercept linear mixed model, with subject as a random effect. Fixed effects for each model consisted of time, Se treatment group and time-by-treatment interactions, with time included as a categorical variable. Between-group comparisons at each time point were assessed within the same model using the time-by-treatment group interaction terms. Pearson's correlations were used to examine associations for variables within each group and associations for variables as combined group. Statistical analyses were two-sided, and a P value of < 0·05 was considered to be statistically significant for within-group and between-group comparisons and for all Pearson's correlations.
Results
Baseline characteristics of subjects and compliance with treatment
A total of twenty-three participants were randomised for the study; all were healthy at the time of commencement of the study. Their mean age was 64 years, ranging from 52 to 79 years. Of these twenty-three participants, twenty-one completed the entire intervention study (fourteen males and seven females) and one withdrew after week 3 due to an inability to tolerate the size of the dairy-Se portion; all other participants tolerated both diary and yeast products. Rectal biopsies of one participant could not be used for PCR due to inadequate quantity of mucosal tissues. There were no significant differences in baseline characteristics between treatment groups (data not shown). There was 100 % compliance with the Se products for those completing the study as estimated by counting the returned sachets at the end of the supplementation.
There were no significant disturbances in plasma glucose or C-reactive protein, or in liver and renal function tests for the duration of the study in any of the participants (Table 2). Consumption of dairy-Se was associated with a non-significant trend towards higher plasma urea in the supplemental period, consistent with compliance with the dairy protein supplement.
Table 2 Effect of selenium supplementation on human plasma glucose, C-reactive protein (CRP), Hb and urea
(Mean values with their standard errors)
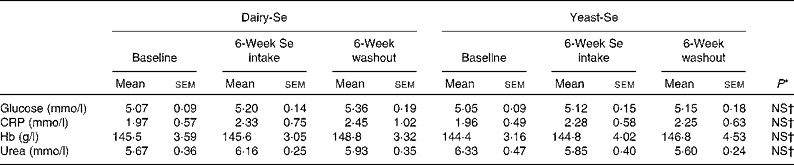
Dairy-Se, Se-enriched milk proteins; yeast-Se, Se-rich yeast.
* Using the Mann–Whitney test.
† Observed values were not significant between dairy-Se and yeast-Se at baseline, after Se supplementation and during the washout period.
Selenium intervention and plasma selenium levels
Plasma Se concentration increased significantly from baseline to between 126 and 135 μg/l (P < 0·001) over the 6-week Se intervention in the two Se groups (Table 3). Plasma Se levels were slightly greater in the yeast-Se group (135 (sem 5·2) μg/l) than in the dairy-Se group (126 (sem 4·0) μg/l) after 6 weeks of Se supplementation (P < 0·05). At the end of the washout period (week 12), plasma Se levels remained significantly elevated, relative to the baseline level (P < 0·01 for dairy-Se and P < 0·001 for yeast-Se), but a steady decline over 6 weeks up to week 12 was apparent. There was no sex effect on plasma Se levels at baseline, after the Se intervention and during the washout period, but women had a slightly higher plasma Se levels than men (Table 4).
Table 3 Effect of selenium-enriched milk proteins (dairy-Se) and selenium-rich yeast (yeast-Se) intervention on plasma selenium concentration and plasma glutathione peroxidase (GPx) activity in human subjects‡
(Mean values with their standard errors)
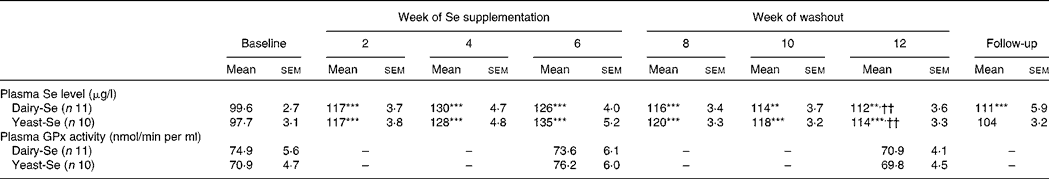
Mean values were significantly different from those of baseline: ** P < 0·01, ***P < 0·001.
†† Mean values were significantly different for week 12 from those of week 6 (P < 0·01).
‡ All comparisons performed using a random-intercept linear mixed model.
Table 4 Effect of sex on plasma selenium concentration and plasma glutathione peroxidase (GPx) activity in human subjects
(Mean values with their standard errors)
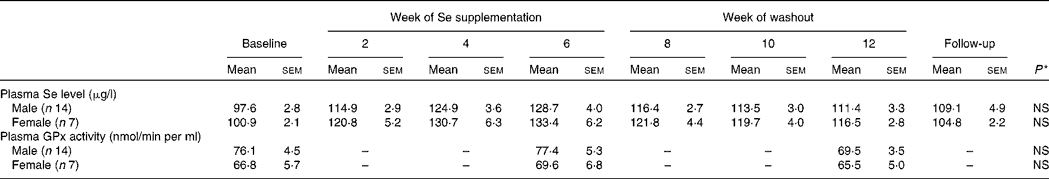
* Observed values were not significant between men and women at baseline, after Se supplementation and during the washout period.
Selenium intervention and plasma glutathione peroxidase activity
Neither dairy-Se nor yeast-Se had significant effects on plasma GPx activity over the 6-week Se intervention (Table 3). Again, there was no sex effect on plasma GPx activity at baseline, after Se supplementation and at the end of the washout period, but men had a trend towards higher plasma GPx activity (Table 4).
Selenium intervention and rectal selenoprotein expression
Changes in rectal selenoprotein gene expression obtained at baseline, after Se intervention and during the washout period were examined in twenty-one participants (Fig. 2). The expression levels of selenoprotein genes differed from each other; the highest level was found in SeP, followed by GPx-2 and GPx-1. TrxR-1 was the least expressed.
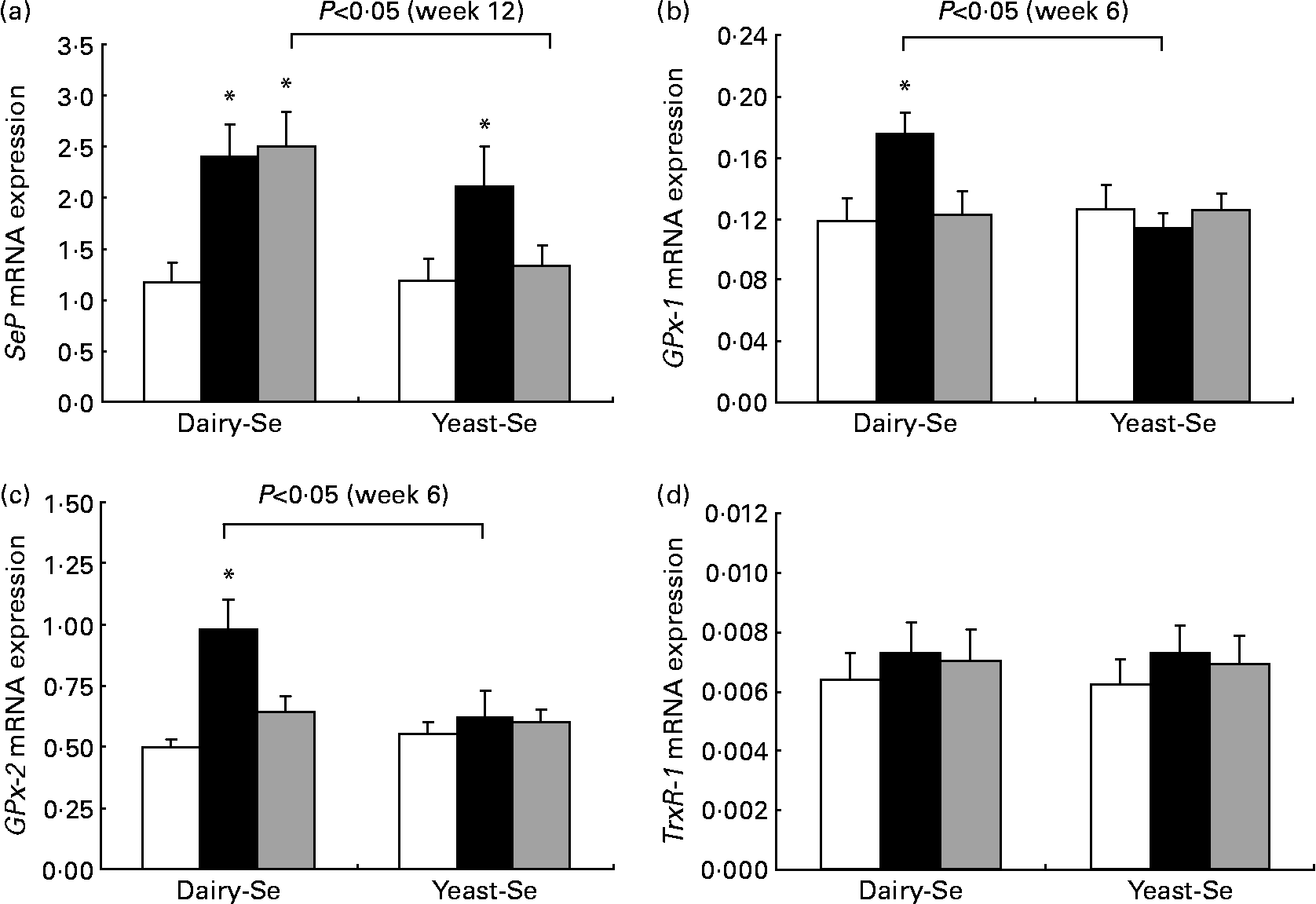
Fig. 2 Quantitative real-time RT-PCR analysis of (a) selenoprotein (SeP), (b) cytosolic glutathione peroxidase-1 (GPx-1), (c) gastrointestinal glutathione peroxidase-2 (GPx-2) and (d) thioredoxin reductase-1 (TrxR-1) mRNA expression in rectal biopsies for Se-enriched milk proteins (dairy-Se, n 11) and Se-rich yeast (yeast-Se, n 10) before Se supplementation (baseline, week 0), after Se supplementation (week 6) and at the end of the washout period (week 12). Gene expression values have been normalised against the reference gene β-actin. Values are means, with their standard errors represented by vertical bars. * Mean values were significantly different compared with the data at week 0 within groups, and brackets indicate differences in data at week 6 or at week 12 between groups (P < 0·05). □, Week 0; ■, week 6; ![]() , week 12.
, week 12.
Following 6 weeks of Se supplementation, the level of SeP mRNA was statistically significantly higher than baseline levels in both Se groups (P < 0·05; Fig. 2(a)). This significant elevation was more sustained in the dairy-Se group compared with the yeast-Se group at the end of the washout period (week 12; P < 0·05). The level of GPx-1 and GPx-2 mRNA expressions at the end of the Se intervention (week 6) was also significantly higher in the dairy-Se group (P < 0·05), but not in the yeast-Se group (Fig. 2(b) and (c)). At week 6, the increase in GPx-2 was greater than that in GPx-1, and at week 12, the level of GPx-2 did not return to the baseline level as rapidly as the level of GPx-1 mRNA. Neither dairy-Se nor yeast-Se significantly affected the TrxR-1 mRNA expression (Fig. 2(d)). Further comparison between the two Se groups showed that the changes in GPx-1 and GPx-2 mRNA at week 6 depended on the Se supplementation (significant P < 0·05 for treatment-by-time interaction), but there was no difference in SeP mRNA at week 12 between the two Se groups. There was no significant sex-specific difference in rectal selenoprotein expression. Rectal SeP and GPx-2 mRNA levels at baseline, after Se supplementation and at the end of the washout period were slightly higher in men than those in women. But the response of SeP, GPx-2 and GPx-1 to Se supplementation and Se withdrawal was similar in men and women (Table 5).
Table 5 Effect of sex on the relative expression of rectal selenoprotein (SeP)* mRNA levels in human subjects
(Mean values with their standard errors)

GPx-1, cytosolic glutathione peroxidase-1; GPx-2, gastrointestinal glutathione peroxidase-2; TrxR-1, thioredoxin reductase-1.
* Gene expression of four SeP genes is related to the expression of β-actin as a reference gene.
† Observed values were not significant between men and women at baseline, after Se supplementation and during the washout period.
Relationship between selenium status and selenoprotein expression
For subjects treated with dairy-Se, changes in plasma Se levels were not correlated with the changes in rectal SeP mRNA at the end of the Se intervention (data not shown), although plasma Se levels did fall within a narrow range. However, the changes in rectal SeP mRNA were positively correlated with the changes in GPx-2 mRNA (r 0·67, P = 0·02; Fig. 3(a)). A positive trend between the changes in rectal SeP mRNA and GPx-1 mRNA was also found (r 0·46, P = 0·10; Fig. 3(b)). Significant correlations were not found in subjects treated with the yeast-Se supplement.
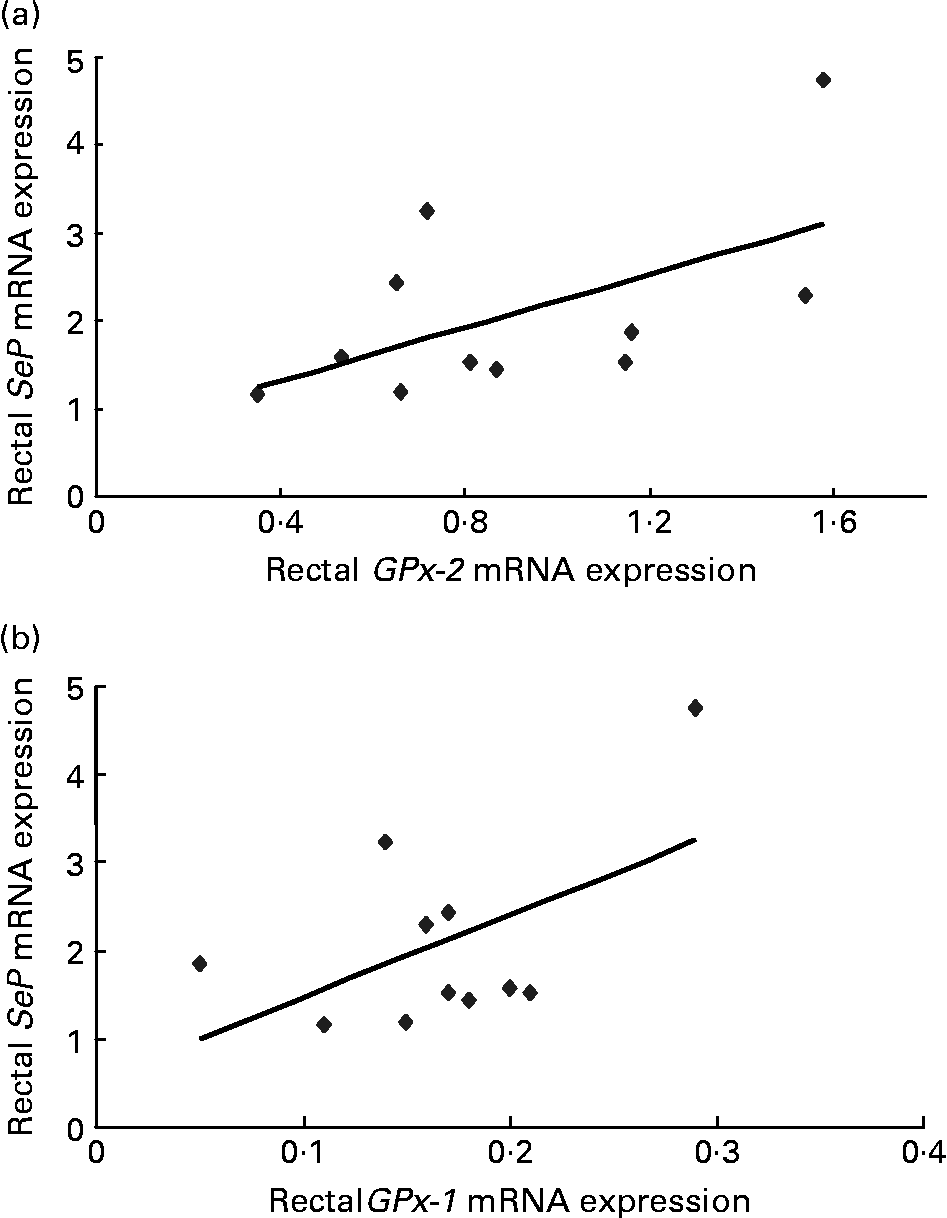
Fig. 3 (a) Correlation between plasma rectal selenoprotein (SeP) mRNA and gastrointestinal glutathione peroxidase-2 (GPx-2) mRNA (r 0·67; P = 0·02) and (b) rectal SeP mRNA and cytosolic glutathione peroxidase-1 (GPx-1) mRNA (r 0·46; P = 0·10) after 6 weeks of Se-enriched milk protein supplementation (n 11, one participant withdrew). There is a positive correlation between the changes in rectal SeP mRNA and GPx-2 mRNA, and a trend towards positive correlation between rectal SeP mRNA and GPx-1 mRNA.
Selenium intervention and rectal epithelial biology
Dietary intervention studies in human subjects have shown that 4–6 weeks on a diet is sufficient to stabilise epithelial kinetics(Reference Macrae, Kilias and Selbie29, Reference Wacker, Wanek and Eder30). Adequate biopsy material, containing a sufficient number of completely sectioned crypts, was available from twenty-one participants before the Se intervention, from twenty participants after the Se intervention and from sixteen participants during the washout period. Neither crypt proliferation nor cell height was significantly affected by Se supplementation (Fig. 4). Occasional spontaneous apoptosis was found in some crypts, but there was no significant difference between dairy-Se and yeast-Se.
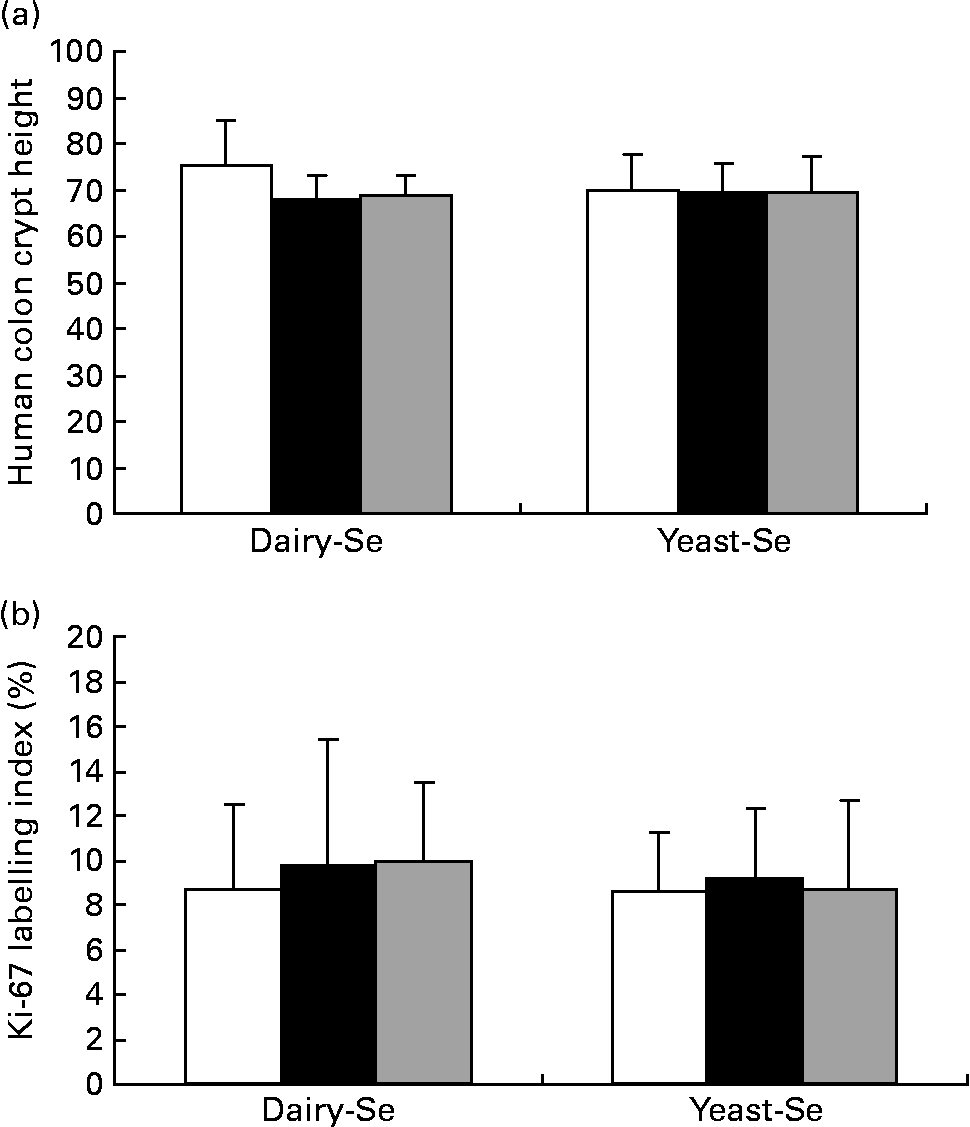
Fig. 4 Effects of dietary Se intervention on (a) crypt height and (b) cell proliferation in rectal biopsies before Se supplementation (baseline, week 0, □), after supplementation (■, week 6) and at the end of the washout period (![]() , week 12). Values are means, with their standard errors represented by vertical bars. Se supplementation has no effects on human rectal cell kinetics. Diary-Se, Se-enriched milk proteins; yeast-Se, Se-rich yeast.
, week 12). Values are means, with their standard errors represented by vertical bars. Se supplementation has no effects on human rectal cell kinetics. Diary-Se, Se-enriched milk proteins; yeast-Se, Se-rich yeast.
Discussion
The present human intervention study investigated the association between organic Se supplementation and selenoprotein gene expression in rectal tissues, because if we are to expect benefit in terms of colorectal disease, we would expect Se supplementation to lead to changes in selenoprotein gene expression in the target tissue. We showed that supplementation of Se (150 μg Se/d) from either dairy or yeast sources resulted in higher rectal SeP mRNA expression, but only dairy-Se was able to significantly increase the expression of rectal GPx-1 and GPx-2 mRNA, and maintain higher SeP mRNA levels during the 6-week washout period. Neither dairy-Se nor yeast-Se regulated rectal TrxR-1 mRNA. We showed that there was no sex effect on plasma Se levels, plasma GPx activity or rectal selenoprotein expression based on the data of fourteen men and seven women. These results support our previous observation in mice that several selenoprotein genes were regulated differently by dairy-Se compared with yeast-Se, when increasing Se intake tenfold beyond nutritional adequacy(Reference Hu, McIntosh and Le Leu20).
Currently, we are unable to explain why the two Se-rich products are significantly different in their influence. According to the Se speciation data available(Reference Rayman27), both products were similar with respect to their predominant components of selenomethionine (83 %) and selenocysteine (5 %), albeit there was a significant component of dairy-Se unidentified. Given the nature of its preparation, dairy-Se did not contain Se compounds smaller than 10 kDa. The present results support the concept that the chemical form of Se and not Se per se is a critical determinant of Se influences on gene expression(Reference Neve31–Reference Facompre and El-Bayoumy33). While it is not clear whether a particular chemical form of Se in dairy protein can significantly influence selenoprotein expression, it is known that the chemical forms of Se in the food will affect Se bioavailability (absorption, retention and utilisation), Se metabolism and the ability to synthesise selenoproteins and to produce methylselenol metabolites(Reference Shiobara, Yoshida and Suzuki34, Reference Medina, Thompson and Ganther35). It is possible that Se in milk proteins is more highly absorbed, differentially metabolised, and/or transported and/or retained by the rectal tissues than with Se from yeast, resulting in an increased expression of rectal molecular markers examined. The present study is limited in the ability to control for protein intake in comparative treatments. Unlike our previous mouse study in which the comparison of dairy-Se and yeast-Se allowed us to balance milk protein concentration (20 %), in the present study, milk protein was substituted into the diet in the place of normal dairy serves. While this is an uncontrolled factor in the study, it represents just a fraction of total protein intake, about one-third to one-fifth of daily protein intake for adult Australians. There has been no evidence that protein concentration in the diet affects Se status, Se metabolism or selenoprotein expression, whereas there has been good evidence in the literature for Se intake influencing selenoproteins(Reference Gromadzinska, Reszka and Bruzelius21). In addition, genetic variants in selenoprotein genes may also account for inter-individual differences in response to Se supplementation(Reference Meplan, Hughes and Pardini36).
Because Se functions through selenoproteins, plasma Se levels and GPx activity are commonly used as indicators of Se status because they respond rapidly to the changes in dietary Se intake(Reference Burk, Norsworthy and Hill37). Selenomethionine is an important factor influencing plasma Se levels, having higher bioavailability and higher retention rates. Since dairy-Se and yeast-Se had a similar selenomethionine concentration and were equally efficient in raising plasma Se levels, this indicates that selenomethionine from both sources was converted to selenocysteine in SeP (approximately 65 % of plasma Se is SeP)(Reference Burk, Norsworthy and Hill37). Our data showed that 6 weeks of supplementation was an adequate time for circulating plasma Se levels to reach a plateau. Interestingly, plasma Se levels remained relatively high during the washout period and had not returned to entry levels at the end of the washout period, particularly for dairy-Se. Similar to our observation, the lack of return of plasma Se to baseline levels during the washout period has also been found after a 6-week supplementation with 100 μg sodium selenite(Reference Meplan, Crosley and Nicol38). Whether this is associated with incorporation of Se into protein pools or retention of Se in other tissues needs to be investigated in future.
The lack of response of plasma GPx activity to both Se supplements implies that subjects in the present study had adequate starting Se levels (mean baseline value 98·6 μg/l) for full expression of plasma GPx activity. The important observation from the present study was the lack of correlation between plasma Se status and rectal selenoprotein gene expression, such that neither plasma Se levels nor plasma GPx activity reflected the biological efficacy of Se supplementation on regulating selenoproteins in target tissues(Reference Ravn-Haren, Krath and Overvad39). A recent Se supplementation study has suggested that plasma Se biomarkers do not mirror Se intake once the Se requirement has been met(Reference Burk, Norsworthy and Hill37), thus there are limitations with the use of plasma Se biomarkers.
Non-specific incorporation of Se into proteins (selenomethionine) is likely to account for the increase in Se levels in tissues, but we did not measure the Se levels in rectal tissues due to limited availability of tissue, nor can we explain why the changes in plasma Se levels were not reflected in changes in rectal selenoprotein gene expression. Others have also reported that whole-blood cell selenoprotein mRNA, such as GPx-1 and SeP, was not significantly correlated with plasma Se in healthy British adults(Reference Sunde, Paterson and Evenson23). It seems likely that specific tissue concentrations of individual selenoproteins can also be used as functional indicators of Se status. In clinical trials, higher Se intake showed protection against cancer without increasing circulating selenoprotein levels(Reference Burk, Norsworthy and Hill37).
SeP is a major plasma selenoprotein (approximately 70 % of plasma Se) and is crucial for Se supply to different organs for the synthesis of other selenoproteins(Reference Hoffmann, Hoge and Li40, Reference Burk and Hill41). However, the level of Se intake required for maximal expression of SeP is not known. Compared with GPx, SeP requires a greater Se intake to reach its maximum concentration; recent studies have suggested that in addition to the habitual intake (approximately 55 μg Se/d), additional intake of 50 μg Se/d is required to achieve the optimal expression of plasma SeP(Reference Hurst, Armah and Dainty24). Plasma SeP has been considered to be a useful biomarker of Se status in populations, suggesting that rectal SeP is also a useful biomarker of Se status. It is likely that subtle alterations in SeP concentration or function would be expected to change Se supply to different tissues and therefore expression of other selenoproteins, such as GPx-1 and GPx-2. Therefore, dietary regulation of rectal SeP expression may have benefits beyond its transport functions.
The present results suggest that GPx-1 and GPx-2 are responsible for 70 % of GPx activity in the gastrointestinal tract(Reference Esworthy, Swiderek and Ho42). A selenoprotein hierarchy, resulting from a competition for available Se and for components of the selenoprotein synthetic machinery, is particularly noticeable in the GPx family, with GPx-1 being the last in the ranking order(Reference Gromadzinska, Reszka and Bruzelius21). For instance, GPx-1 mRNA fell dramatically in Se deficiency, whereas GPx-2 mRNA was remarkably stable (conserved) and increased rapidly on Se supplementation, indicating the biological importance of GPx-2 in the gastrointestinal tract(Reference Rayman22). To date, most studies have focused on Se deficiency on selenoprotein expression(Reference Pagmantidis, Bermano and Villette43, Reference Irons, Carlson and Hatfield44), whereas there are limited data of Se supplementation on the regulation of selenoproteins in human subjects(Reference Meplan, Nicol and Burtle45). In the present study, dairy-Se supplement resulted in a greater increase of rectal GPx-2 mRNA than GPx-1 mRNA, which may, in part, be associated with GPx-2 tissue specificity and stability in Se deficiency (i.e. selenoprotein hierarchy). Since GPx-1 and GPx-2 have diverse biological roles and are implicated in the risk and the development of human CRC(Reference Mork, Al-Taie and Bahr46–Reference Diwadkar-Navsariwala and Diamond48), this up-regulation may have health benefits for humans. It is likely that the anti-cancer property of Se is mediated by specific selenoproteins in the gastrointestinal tract.
TrxR-1, as part of the thioredoxin system, is important in antioxidant defence and is relevant to anti-cancer function(Reference Moghadaszadeh and Beggs49). Similar to our mouse study, TrxR-1 mRNA did not respond to the Se supplements. Al-Taie et al. (Reference Al-Taie, Seufert and Karvar50) also reported that Se had no effect on TrxR, so more research is required to demonstrate the roles of TrxR-1 and its regulation in human rectal epithelium.
While diabetes risk was not a primary study endpoint, an additional observation from the present study was that there was no alteration in any markers of diabetes risk, as had been proposed by some researchers(Reference Laclaustra, Navas-Acien and Stranges51). Interestingly, a recent French study has shown the opposite effect: a decreased risk of diabetes in subjects with increasing Se status(Reference Akbaraly, Arnaud and Rayman52). Further studies are needed to determine whether any association exists between Se supplementation and diabetes risk.
In conclusion, the present study indicates for the first time that rectal selenoprotein gene expression (i.e. SeP, GPx-1 and GPx-2) is significantly regulated by dietary Se supplementation independent of plasma levels of Se and GPx activity; furthermore, regulation depends on the source/form of dietary Se supplementation; dairy-Se had a more sustained effect. Up-regulation of rectal SeP, GPx-1 and GPx-2 raises the potential for Se supplementation to directly influence the risk for colorectal disease. Therefore, a novel form of Se such as this dairy-sourced Se warrants further investigation to test this possibility, especially in relation to the possibility of reducing the risk of CRC.
Acknowledgements
The present study was funded by the Gardiner Foundation Major Research and Development Project, Melbourne, VIC, Australia (project no. MP4/009), National Health and Medical Research Council of Australia (project no. 324719, Canberra, Australia) and Cancer Council SA, Eastwood, SA, Australia (project no. 480430, Adelaide, Australia). Thanks to all the volunteers who generously gave their time for the present study. The authors thank the support from the Department of Primary Industries Victoria, Tatura Milk Industries Limited, Alltech Biotech Private Limited, the Geoffrey Gardiner Foundation, Melbourne, VIC, Australia. Y. H., G. H. M. and G. P. Y. were involved in the design of the study. Y. H., R. K. L. L. and J. M. U. were responsible for the execution of the experimental work and data collection. Y. H. and R. J. W. were responsible for the data analysis. Y. H. was responsible for writing the manuscript. None of the authors has a conflict of interest.













