Type 2 (non-insulin-dependent) diabetes mellitus is one of the most common metabolic diseases in humans, affecting about 3 % of the human population. Clinically, it is rather a group of related diseases, characterised by chronic hyperglycaemic levels, due to an impairment in the balance among the three processes of β-cell secretion of insulin, peripheral resistance to insulin and hepatic glucose production(Reference Biessels, Kappelle and Bravenboer1, Reference McCarty2). Depending on several factors, such as obesity, age of onset, mode of inheritance and severity of glucose intolerance, clinical features of individuals suffering from type 2 diabetes are highly variable.
Despite being one of the most common non-communicable diseases in the world, the exact mechanism (or mechanisms) leading to glucose intolerance is still not clear(Reference Biessels, Kappelle and Bravenboer1, Reference Zimmet3). Recently, mitochondrial dysfunction due to oxidative damage has become known as a major contributor to ageing, to degenerative diseases such as cancer, and to the metabolic syndrome, obesity and type 2 diabetes(Reference Ames, Shigenaga and Hagen4, Reference Lowell and Shulman5). A group of antioxidants/metabolites which can act to improve mitochondrial function and protect mitochondria from oxidative damage, when supplemented in the diet, have been defined as mitochondrial nutrients(Reference Cai, Jia and Liu6, Reference Liu, Shen and Zhao7). One good example of a mitochondrial nutrient is R-α-lipoic acid (LA)(Reference Liu, Shen and Zhao7–Reference Liu9). Here, the terms nutrients or mitochondrial nutrients denote the combination of LA, acetyl-l-carnitine, nicotinamide and biotin.
The Goto–Kakizaki (GK) rat is known as a model of type 2 diabetes. The pathogenesis of diabetes in GK rats involves insulin resistance, an abnormal glucose metabolism as well as an impaired ontogenetic development of pancreatic islet cells(Reference Janssen, Vassiliadou and Riley10, Reference Portha, Lacraz and Kergoat11) and mitochondrial dysfunction in the liver(Reference Palmeira, Ferreira and Santos12). This model is particularly relevant to the understanding of human type 2 diabetes because mild hyperglycaemia has been demonstrated as early as 2–4 weeks after birth in these animals. Until 12 months of age, GK rats exhibit a moderate and stable fasting hyperglycaemia, which does not progress to a ketotic state. Therefore, at an early stage of diabetes, the GK rat does not present the severe complications associated with this condition. It is thus an appropriate model to study events occurring at the onset of diabetes, compared with obese diabetic rats which present severe hyperglycaemia and hyperlipidaemia(Reference Duhault and Koenig-Berard13).
Treatment of GK rats with mitochondrial nutrients significantly improves glucose tolerance, insulin release, plasma NEFA levels, skeletal muscle mitochondrial biogenesis(Reference Shen, Hao and Tian14) and immune function(Reference Hao, Shen and Tian15). The rationale for the selection and dosage levels of these nutrients, as detailed earlier(Reference Shen, Hao and Tian14, Reference Hao, Shen and Tian15), targets substances that influence mitochondrial processes either synergistically or by mechanisms as widely disparate as possible. Briefly, acetyl-l-carnitine and LA were selected for the ability of each to ameliorate mitochondrial dysfunction: acetyl-l-carnitine by its ability to facilitate the transport of long-chain fatty acids across the mitochondrial membrane and LA by its ability to improve insulin sensitivity by adenosine monophosphate-activated protein kinase (AMPK) activation. Both nutrients were included because the combination is more effective than either nutrient administered alone. Niacin was added for its ability to lower plasma glucose, plasma NEFA and hepatic glucose production, and for its enhancement of insulin-stimulated glucose uptake in streptozotocin-treated rats. Finally, biotin was selected based on its role as a cofactor for four mitochondrial carboxylases, activities of which are thought to be normalised by a combination of biotin and LA.
The liver is one of the major organs affecting and being affected by diabetes and hyperglycaemia. Excessive gluconeogenesis in the liver contributes to hyperglycaemia(Reference Gastaldelli, Baldi and Pettiti16), and chronically elevated blood glucose has a significant impact on the liver itself. Diabetic patients have been found to have excessive glycogen deposition in the liver(Reference Clore, Post and Bailey17) and excessive build-up of fat in the liver as steatosis(Reference Lonardo, Lombardini and Ricchi18). More recently, Almon et al. (Reference Almon, DuBois and Lai19) compared gene expression patterns in the liver of Wistar and GK rats and found that 311 genes are differentially expressed in the liver of the two strains. A functional analysis of these genes indicated that disruption of lipid metabolism in the liver is a major consequence of chronic hyperglycaemia in the GK strain and that chronic inflammation contributes significantly to the development of diabetes in GK rats. In the present study, the effects of treatment with mitochondrial nutrients on mitochondrial function, oxidative damage and apoptosis in the liver of GK rats are presented, and compared with those of pioglitazone treatment.
Materials and methods
Animals and treatments
Male diabetic GK rats, 4 weeks old, together with age-matched male non-diabetic Wistar rats were purchased from SLAC Laboratory Animal Company Limited (Shanghai, China). All animals were housed at 23 ± 2°C under 12 h light–12 h dark cycles, and allowed access to food and water ad libitum. National Institutes of Health(20) principles of laboratory animal care were strictly followed, and all experimental procedures were approved by the Institutional Animal Use and Care of the Institute for Nutritional Science, Shanghai Institutes of Biological Sciences.
Rats were divided into four groups (each group, n 10): (1) untreated Wistar and (2) GK control groups, (3) a pioglitazone group that received pioglitazone (20 mg/kg per d) by oral administration, (4) a nutrient group that received a combination of R-α-LA (50 mg/kg per d), acetyl-l-carnitine (100 mg/kg per d), biotin (0·1 mg/kg per d) and nicotinamide (15 mg/kg per d) by oral administration. The control groups received the same oral administration volume of PBS alone. The treatments started at 6 weeks of age, and continued for 3 months. At approximately 18 weeks of age, animals were anaesthetised with an intraperitoneal injection of sodium pentobarbital (60 mg/kg) and killed in order to obtain the liver.
Isolation of liver mitochondria and liver tissue protein
Liver mitochondria were isolated as described previously(Reference Krahenbuhl, Chang and Brass21) with a slight modification(Reference Long, Wang and Gao22, Reference Long, Gao and Sun23). Mitochondrial protein concentration was determined using a Pierce BCA™ Protein Assay kit (product no. 23225; Thermo Scientific, Rockford, IL, USA), with bovine serum albumin as a standard. Mitochondria were stored at − 80°C until enzyme analysis.
For tissue protein collection, approximately 100 mg of liver samples were homogenised in ice-cold solubilisation buffer containing 65 mm-Tris (pH 7·4), 150 mm-NaCl, 5 mm-EDTA, 1 % Nonidet P-40, 0·5 % sodium deoxycholate, 0·1 % SDS, 10 % glycerol, aprotinin (1 μg/ml), leupeptin (1 μg/ml), 10 mm-sodium fluoride, 1 mm-Na3VO4 and 1 mm-phenylmethylsulfonylfluoride. Protein concentrations were determined as described earlier.
Assays of complexes I, II, III, IV and V activities in mitochondria
The activities of the mitochondrial complexes NADH–coenzyme quinone (CoQ) oxidoreductase (complex I), succinate–CoQ oxidoreductase (complex II), CoQ–cytochrome c reductase (complex III), cytochrome c oxidase (complex IV) and ATP synthase (complex V) were measured spectrometrically as described previously(Reference Sun, Luo and Long24–Reference Jia, Liu and Sun26).
Assays for activities of total antioxidant capacity, superoxide dismutase, glutathione S-transferase, NAD(P)H:quinone oxidoreductase 1 and reduced glutathione in mitochondria
Total antioxidant capacity was assayed with a commercially available assay kit (Jiancheng Biochemical, Inc., Nanjing, China). The principle of the test is to measure the change in colour upon conversion of Fe3+ to Fe2+ by the reducing components in the samples. Optical density was measured at 520 nm with a microplate reader. Total superoxide dismutase activity was assayed using a commercially available SOD Detection Kit (Jiancheng Biochemical, Inc.). The assay is based on the superoxide generated by the xanthine/xanthine oxidase system. Optical density at 550 nm was measured with a microplate reader. Glutathione S-transferase activity was measured with 2 μg protein in a final 200 μl volume of 1 mm-reduced glutathione (GSH), 1 mm-chloro-2,4-dinitrobenzene, and bovine serum albumin (3 mg/ml) in 10 mm-sodium phosphate buffer. The mixture was scanned at 340 nm for 5 min at 25°C as described previously(Reference Pabst, Habig and Jakoby27).
NAD(P)H:quinone oxidoreductase 1 activity was measured with 10 μg protein in a final 200 μl volume of 50 mm-Tris–HCl (pH 7·4), 80 μm-dichlorophenolindophenol (DCPIP), bovine serum albumin (1 mg/ml), 0·005 % Tween-20 (v/v), and 0 or 10 μm-dicoumarol; the reaction was initiated with 180 μm-NADPH. The reduction of DCPIP was scanned at 600 nm at 25°C with a microplate reader. NAD(P)H:quinone oxidoreductase 1 activity was considered to be the dicoumarol-inhibitable part of the DCPIP reduction.
GSH content was determined in the liver with a commercially available GSH Detection Kit (Jiancheng Biochemical, Inc.) that utilises a thiol-specific reagent, dithionitrobenzoic acid. The adduct was measured spectrophotometrically at 412 nm.
Western blot of glyceraldehyde 3-phosphate dehydrogenase in mitochondria
Liver mitochondrial protein samples (10 μg) were subjected to 10 % SDS-PAGE; proteins were then transferred to nitrocellulose membranes and blocked with 5 % non-fat milk/TBST (Tris-buffered saline Tween 20) for 1 h at room temperature. Membranes were incubated with primary monoclonal antibodies directed against glyceraldehyde 3-phosphate dehydrogenase (GAPDH, 1:1000) overnight at 4°C. Western blots were developed using enhanced chemiluminescence (Roche, Mannheim, Germany) and quantified by scanning densitometry. As mitochondrial protein loading controls, polyacrylamide resolving gels (10 %, w/v) loaded with the same samples were stained with Coomassie Brilliant Blue R250.
Detection of mitochondrial protein carbonyls
Protein carbonyls in liver mitochondria were assayed with the Oxyblot protein oxidation detection kit (Chemicon, Temecula, CA, USA)(Reference Sun, Luo and Long24, Reference Levine, Garland and Oliver28).
Western blot analysis of p53 and p21
Liver tissue proteins were subjected to 10 % SDS-PAGE and detected with primary antibodies against p53 (1:1000, Mouse Ab no. 12 506; Santa Cruz Biotechnology, Santa Cruz, CA, USA), p21 (1:1000, Mouse Ab no. 2946; Cell Signaling Technology, Danvers, MA, USA) or α-tubulin (1:5000). Western blots were developed using enhanced chemiluminescence (Roche) and quantified by scanning densitometry (Bio-Rad Laboratories, Hercules, CA, USA) after incubation with horseradish peroxidase-conjugated secondary antibody.
Determination of NEFA contents of Goto–Kakizaki liver tissue
NEFA contents of supernatants were estimated by a commercially available NEFA kit (Jiancheng Biochemical, Inc.). The test is based on the reaction of NEFA with Cu2+ to form Cu salts, which are detected photometrically at 440 nm.
RNA isolation and RT-PCR for PPARα and carnitine palmitoyltransferase I
Total RNA was isolated using the single-step TRI-reagent (TRIzol), and 1 μg of isolated RNA was then reverse-transcribed into complementary DNA. Primer sequences used for quantification of mRNA by real-time quantitative PCR for PPARα, carnitine palmitoyltransferase I (CPT-1), and 18S ribosomal RNA and mRNA have been described previously(Reference Shen, Liu and Tian29).
Statistical analysis
Data are presented as means with their standard errors. Statistical significance was calculated with SPSS 10.0 software (SPSS Inc., Chicago, IL, USA) using one-way ANOVA. P values < 0·05 were considered significant.
Results
Activities of liver mitochondrial complexes
As shown in Fig. 1, all five complexes composing the respiratory chain were assayed photometrically. Compared with Wistar controls, activities of complexes I, III and IV distinctly decreased in the liver mitochondria of GK rats (P < 0·01). Conversely, the activities of complexes II and V distinctly increased in GK rats. The nutrient treatment significantly prevented the decreases in complexes I, III and IV (P < 0·05); in contrast, treatment with pioglitazone produced no significant improvement. As for complexes II and V, nutrient treatment showed no significant changes compared with GK untreated rats, while pioglitazone treatment significantly increased the activity of complex V.

Fig. 1 Liver mitochondrial activities of complexes I–V. The complex activities were determined as described in Materials and methods. Results are expressed as fold change of control. Values are means, with their standard errors represented by vertical bars (n 10). Mean value was significantly different from that of the Wistar group: * P < 0·05, ** P < 0·01. † Mean value was significantly different from that of the untreated Goto–Kakizaki (GK) group (P < 0·05). □, Wistar; ■, GK; ![]() , GK with nutrients;
, GK with nutrients; ![]() , GK with pioglitazone.
, GK with pioglitazone.
Liver mitochondrial protein carbonyl levels
As shown in Fig. 2, protein carbonyl levels were higher in the liver mitochondria of GK rats compared with those of control rats (P < 0·01). Nutrient treatment significantly diminished the carbonyls in liver mitochondria compared with GK rats (P < 0·05), while in the pioglitazone group, there was only a tendency for carbonyl levels to be lowered in liver mitochondria.
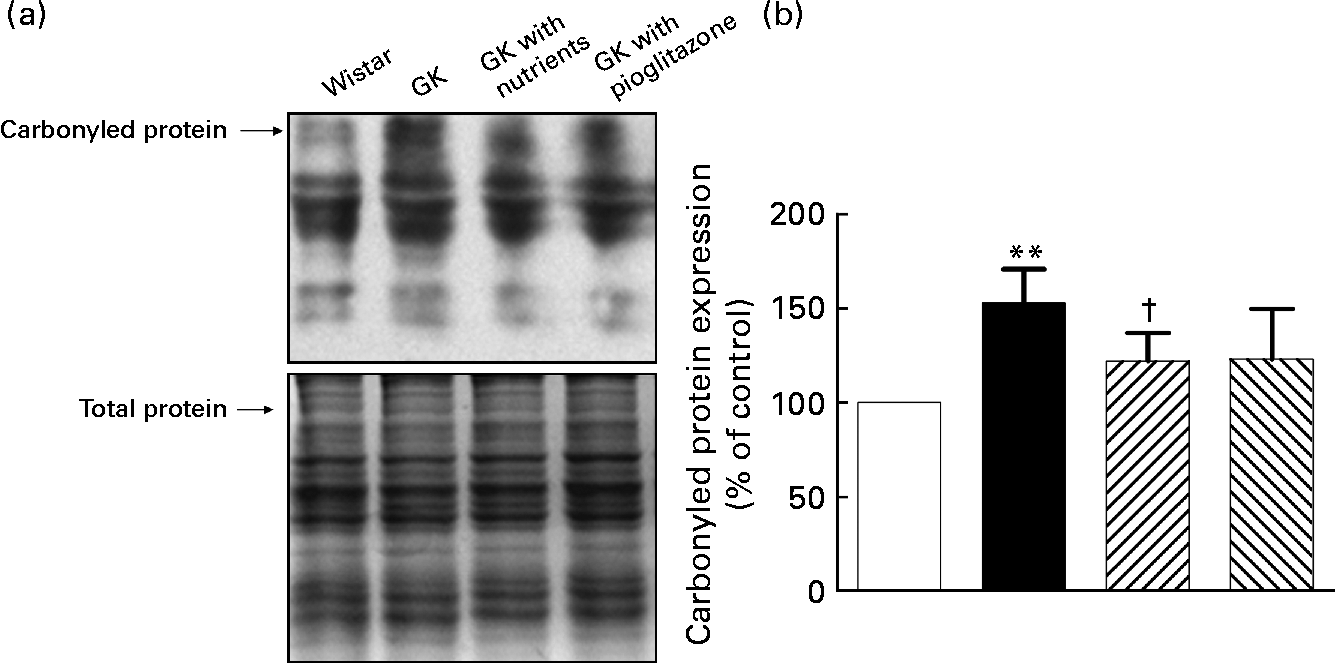
Fig. 2 Carbonylated protein levels in liver mitochondria. Liver protein carbonyls were detected by Western blotting using the Oxyblot protein oxidation detection kit (Chemicon, Temecula, CA, USA). The second set of polyacrylamide resolving gels (12 %, w/v) loaded with the same quantity of samples was electrophoresed and stained with Coomassie Brilliant Blue R250 as a loading control. (a) Western blotting images of carbonylated protein; (b) quantitative results of carbonylated protein. Quantitative values were computed as the ratio of band densities of the respective protein to total balanced protein. Results are expressed as fold change of control. Values are means, with their standard errors represented by vertical bars (n 10). ** Mean value was significantly different from that of the Wistar group (P < 0·01). † Mean value was significantly different from that of the untreated Goto–Kakizaki (GK) group (P < 0·05). □, Wistar; ■, GK; ![]() , GK with nutrients;
, GK with nutrients; ![]() , GK with pioglitazone.
, GK with pioglitazone.
Total antioxidant capacity, reduced glutathione contents and activities of total superoxide dismutase, glutathione S-transferase and NAD(P)H:quinone oxidoreductase 1
The total antioxidant capacity of the GK rat liver was distinctly reduced (Fig. 3(a)), and treatment of nutrients significantly elevated it; however, the pioglitazone group did not show a significant increase. In contrast, the GSH level in the GK rat liver unexpectedly increased, and treatment with nutrients and pioglitazone tended to normalise it (Fig. 3(a)). Compared with the Wistar control mitochondria, activities of total superoxide dismutase, glutathione S-transferase and NAD(P)H:quinone oxidoreductase 1 were significantly reduced in GK rats (P < 0·05), and the nutrient-treated group demonstrated significant changes as shown in Fig. 3(b).

Fig. 3 Liver mitochondrial total antioxidant capacity (T-AOC), contents of reduced glutathione (GSH) and activities of total superoxide dismutase (T-SOD), glutathione S-transferase (GST) and NAD(P)H:quinone oxidoreductase 1 (NQO1). (a) T-AOC and GSH in mitochondria (□, Wistar; ■, Goto–Kakizaki (GK); ![]() , GK with nutrients;
, GK with nutrients; ![]() , GK with pioglitazone) and (b) activity of T-SOD, GST and NQO1 in mitochondria (□, Wistar; ■, GK;
, GK with pioglitazone) and (b) activity of T-SOD, GST and NQO1 in mitochondria (□, Wistar; ■, GK; ![]() , GK with nutrients;
, GK with nutrients; ![]() , GK with pioglitazone). Results are expressed as fold change of control. Values are means, with their standard errors represented by vertical bars (n 10). Mean value was significantly different from that of the Wistar group: * P < 0·05, ** P < 0·01. † Mean value was significantly different from that of the untreated GK group (P < 0·05).
, GK with pioglitazone). Results are expressed as fold change of control. Values are means, with their standard errors represented by vertical bars (n 10). Mean value was significantly different from that of the Wistar group: * P < 0·05, ** P < 0·01. † Mean value was significantly different from that of the untreated GK group (P < 0·05).
Protein expression of glyceraldehyde 3-phosphate dehydrogenase in liver mitochondria
GAPDH protein expression was significantly increased in the liver mitochondria of untreated GK rats compared with the expression in Wistar rat mitochondria (P < 0·05); the nutrient and pioglitazone groups demonstrated significant normalisation of protein expression compared with the GK group (P < 0·05), as shown in Fig. 4.

Fig. 4 Protein expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) in liver mitochondria: (a) Western blotting images of GAPDH protein and (b) quantitative results for GAPDH. Quantitative values were computed as the ratios of the density of the targeted protein to total balanced protein. Results are expressed as fold change of control. Values are means, with their standard errors represented by vertical bars (n 10). * Mean value was significantly different from that of the Wistar group (P < 0·05). † Mean value was significantly different from that of the untreated Goto–Kakizaki (GK) group (P < 0·05). □, Wistar; ■, GK; ![]() , GK with nutrients;
, GK with nutrients; ![]() , GK with pioglitazone.
, GK with pioglitazone.
Protein expression of the apoptosis-related factors, p53 and p21 in liver tissue
p53 protein expression was significantly increased in the liver tissue of GK rats (Fig. 5) compared with that in Wistar rats, and the increase in p53 was accompanied by an increased expression of p21 protein. Nutrient treatment tended to inhibit the increase in p53 and p21, while treatment with pioglitazone did not produce a significant difference.

Fig. 5 Apoptosis-related factors p53 and p21 in liver tissue: (a) Western blotting images of p21, p53 and α-tubulin, respectively and (b) quantitative results of p53 and p21. Quantitative values were computed as the ratios of the density of the targeted protein to α-tubulin. Results are expressed as fold change of control. Values are means, with their standard errors represented by vertical bars (n 10). Mean value was significantly different from that of the Wistar group: * P < 0·05, ** P < 0·01. □, Wistar; ■, GK; ![]() , GK with nutrients;
, GK with nutrients; ![]() , GK with pioglitazone.
, GK with pioglitazone.
NEFA content in the liver
As shown in Fig. 6, NEFA content was higher in the liver of diabetic GK rats than that in Wistar rats; treatment with nutrients significantly decreased the NEFA level (P < 0·05).

Fig. 6 NEFA content in liver tissue. For tissue protein collection, approximately 50 mg of liver samples were homogenised in ice-cold buffer as described in Materials and methods. After centrifugation, the supernatant was used for NEFA detection. Results are expressed as percentage of control. Values are means, with their standard errors represented by vertical bars. * Mean value was significantly different from that of the Wistar group (P < 0·05). † Mean value was significantly different from that of the untreated Goto–Kakizaki (GK) group (P < 0·05). □, Wistar; ■, GK; ![]() , GK with nutrients;
, GK with nutrients; ![]() , GK with pioglitazone.
, GK with pioglitazone.
PPARα and CPT-1 gene expression changes in liver tissue
Levels of PPARα mRNA were lower in GK rats, although the difference was not statistically significant. Administration of nutrients to GK rats significantly increased the expression of PPARα levels (Fig. 7). In contrast, levels of CPT-1 mRNA were significantly lower in GK rats than in Wistar rats (P < 0·05). Administration of either nutrients or pioglitazone to GK rats significantly increased the levels of CPT-1 in the GK rat liver (Fig. 7).

Fig. 7 Gene expression of PPARα and CPT-1, two genes involved in fatty acid oxidation in liver tissue. PCR fluorescence products were quantified using SYBR Green. The cycle number at which the various transcripts were detectable was compared with that of 18S ribosomal RNA as an internal control. Results are expressed as percentage of control. Values are means, with their standard errors represented by vertical bars. * Mean value was significantly different from that of the Wistar group (P < 0·05). † Mean value was significantly different from that of the untreated Goto–Kakizaki (GK) group (P < 0·05). □, Wistar; ■, GK; ![]() , GK with nutrients;
, GK with nutrients; ![]() , GK with pioglitazone.
, GK with pioglitazone.
Discussion
As with β-cells, the liver is highly exposed to the possibility of glucose injury (glucose toxicity). Emerging evidence supports the hypothesis that insulin responsiveness of the liver in type 2 diabetes is caused by mitochondrial dysfunction(Reference Lowell and Shulman5). It has been shown that liver mitochondrial function appears to be more efficient and that the liver has higher levels of antioxidants such as vitamin E, GSH and CoQ in 6-month-old GK rats than in non-diabetic control rats(Reference Ferreira, Palmeira and Matos30–Reference Ferreira, Seica and Oliveira33). It is difficult to understand why GK rat liver mitochondria function at high efficiency while operating under the stressful conditions of diabetic hyperglycaemia. One possible explanation for over-reactive mitochondria and high antioxidant response is a diabetes-induced metabolic maladaptation(Reference Ferreira, Seica and Oliveira33).
In the present study, liver mitochondrial function is significantly altered in GK rats, as indicated by the decreases in complex I, III and IV activities and increases in complex II and V activities. Treatment with the combination of mitochondrial nutrients, more significantly than with pioglitazone, normalised the activity of liver mitochondrial complexes in GK rats towards that in non-diabetic Wistar rats, suggesting that the mitochondrion is a possible target of these nutrients. That complexes I, III and IV all respond similarly is consistent with other characteristics they have in common: the activities of all three are inhibited by NO(Reference Carreras, Franco and Peralta34), critical subunits of all three are encoded by mitochondrial genes (in contrast to complex II) and all three act to pump protons out of the mitochondrial matrix (in contrast to complex V). The increases in complexes II and V are consistent with a previous report by Ferreira et al. (Reference Ferreira, Palmeira and Seica31) but the changes in complexes I and IV are different. Ferreira et al. (Reference Ferreira, Palmeira and Seica31) showed that complex I and complexes II and III did not change while complex IV increased. The lack of change of complexes II and III in Ferreira et al.'s work may be due to an increase in complex II combined with a decrease in complex III, but the reasons for the differences in complexes I and IV between the present study and Ferreira et al.'s are unknown. The only difference between the two studies is that in the present study, nutrients were administered by oral administration, whereas Ferreira et al. used animals without any treatment. Whether stress induced by oral administration caused a decrease in the activity of complexes I and IV warrants further study.
GSH, CoQ and α-tocopherol are important endogenous antioxidants, and are generally decreased by oxidative insult. GK rat liver mitochondria have increases in mitochondrial CoQ(Reference Ferreira, Seica and Oliveira33), vitamin E (α-tocopherol) contents and reduced:oxidised glutathione ratios(Reference Ferreira, Palmeira and Matos30), possibly in response to increased oxidative stress. In agreement with these results, liver GSH levels in GK rats were significantly increased, compared with those in Wistar rats. Treatments with nutrients or pioglitazone reversed the GSH increase by a similar amount, suggesting that they may play a role in relieving hyperglycaemia-induced oxidative stress in the liver. However, it should be pointed out that the lack of the measurement of oxidised glutathione is a limitation in the present study.
GAPDH expression was prominently higher in GK rats, compared with that in Wistar rats (Fig. 4). GAPDH was first characterised as a glycolytic dehydrogenase, but it has been shown since to exert several functions as diverse as apoptosis induction, transfer RNA export, DNA repair or as a receptor-associated kinase(Reference Tarze, Deniaud and Le Bras35). It is a pleiotropic enzyme that is overexpressed during apoptosis and in several human chronic pathologies. This protein accumulates in mitochondria during apoptosis, and regulates pro-apoptotic mitochondrial membrane permeabilisation, a decisive event in the intrinsic pathway of apoptosis. This latter functionality provided motivation to investigate protein expression of the apoptosis-regulating factors p53 and p21. Consistent with the elevation of GAPDH and mitochondrial dysfunction, levels of both p53 and p21 proteins were elevated – the first report of increased expression of these factors in the GK rat liver. Treatment with either the nutrient combination or pioglitazone significantly decreased GAPDH expression (Fig. 4). These treatments also produced a non-significant decrease in p53 and p21 (Fig. 5), consonant with the statistically significant reduction in these two proteins seen in GK rat thymocytes reported earlier(Reference Hao, Shen and Tian15).
As evidence indicating a possible increase in oxidative stress and consequent cell death/tissue damage in GK rat mitochondria, amounts of oxidised proteins were clearly elevated in GK rats, and total antioxidant capacity, total superoxide dismutase, glutathione S-transferase and NAD(P)H:quinone oxidoreductase 1 activities were substantially decreased. These results suggest that hyperglycaemia-induced metabolic maladaptation will consequently cause oxidative damage in GK rats. Treatment of GK rats with mitochondrial nutrients efficiently prevented oxidative damage in liver. These results are in line with the improvement of impaired glucose tolerance and hyperinsulinaemia(Reference Shen, Hao and Tian14), and closely parallel similar ameliorative effects of these nutrients on the same oxidative parameters in GK rat thymocytes(Reference Hao, Shen and Tian15).
NEFA content in diabetic GK rat plasma is substantially elevated(Reference Bogacka, Xie and Bray36), although GK rats demonstrate neither severe dyslipidaemia nor fatty liver. In the present study, NEFA content in the liver tissue of GK animals increased significantly compared with that of Wistar animals, and nutrient treatment significantly ameliorated this increase (Fig. 6). This pattern parallels previously reported results in plasma(Reference Shen, Hao and Tian14). Nutrient treatment also affected the expression of hepatic genes related to lipid oxidation pathways that are altered in GK animals. Expression of PPARα and CPT-1 gene expression decreased in the GK rat liver. PPARα and CPT-1 regulate the mitochondrial and peroxisomal fatty acid β-oxidation systems as well as the microsomal ω-oxidation system. Defects in these factors have been reported to lead to defects in the utilisation of hepatic fat as an energy source and consequently to hypoketonaemia that has a prominent impact on the pathogenesis of fatty liver disease(Reference Reddy and Rao37, Reference Hashimoto, Cook and Qi38). In the GK model, NEFA accumulation was suppressed by mitochondrial nutrient treatment along with significant up-regulation of PPARα and CPT-1 mRNA transcription, indicating that mitochondrial nutrients decreased NEFA contents by up-regulating lipid oxidation pathways. In GK rat muscle, comparable decreases in PPARα and CPT-1 gene expression levels that were restored to wild-type levels by nutrient treatment suggest that similar metabolic alterations may occur in the latter tissue as well(Reference Shen, Hao and Tian14).
In summary, GK rat livers differ from Wistar controls by displaying abnormal changes in the activities of mitochondrial complexes, an increase in oxidative stress, a decrease in antioxidant defences and increases in NEFA levels and apoptosis. Furthermore, a 3-month treatment with a combination of four nutrients ameliorated these abnormalities to the same degree as did treatment with the antidiabetic drug pioglitazone. These results suggest that dietary supplementation with these nutrients may be an effective strategy to improve liver dysfunction in GK diabetic rats by improving mitochondrial function, inhibiting oxidative damage and reducing apoptosis.
Acknowledgements
The authors thank Xu Jia, Lu Zhu, Cheng Luo and Xuesen Li for technical assistance. The present study was supported by Pujiang Talent Award (05PG14104), the Open Research Fund Program of Key Laboratory of Marine Drugs Ministry of Education, Ocean University of China, a grant from the Shanghai Science and Technology Committee (074319105), Shanghai, China, the National Natural Science Foundation of China Grant (30871002), a UC Davis Center for Human and Nutrition Pilot Award (CHNR08-318) and the financial support of 985 and 211 projects of Xi'an Jiaotong University and the National Natural Science Foundation of China (grant no. 31070740). J. H., W. S., X. S. and J. L. conceived and designed the experiments; J. H., W. S., L. S. and J. L. performed the experiments; J. H., W. S. and J. L. analysed the data; J. H., E. S. and J. L. wrote the manuscript. A patent (application no. 200710046615.8) has been filed on the combination of nutrients mentioned in the study. The authors declare that no competing interests exist.











