- CWG
conditional weight gain
- GMS
Gateshead Millennium Study
- IEAS
infancy eating avidity score
With rising rates of obesity at ever earlier ages, the importance of childhood as a period when obesity could be prevented or reversed has grown. Within childhood no clear onset age has been identified, leading some researchers to argue that interventions to prevent childhood obesity should begin in the toddler years or even infancy(Reference Paul, Bartok and Downs1). It has been proposed that the duration of breast-feeding and timing of first solids is related to later obesity(Reference Schack-Nielsen, Sorensen and Mortensen2), so that general interventions to promote breast-feeding and defer solids are now also commonly regarded as important for obesity prevention. Accelerated infant weight gain has been associated with later childhood obesity(Reference Baird, Fisher and Lucas3), which might suggest that infants identified as at ‘high risk’ for onset of obesity in the first year could be targeted for early intervention(Reference Barlow, Whitlock and Hanson4).
A number of studies in childhood have found relationships between child eating behaviour and overweight or adiposity(Reference Carnell and Wardle5–Reference Johnson and Birch8). Recent studies have proposed that distinctive childhood eating behaviours related to overweight are heritable, suggesting that at least some of the genetic predisposition to obesity reflects an inherent tendency to overeat(Reference Carnell and Wardle9). Thus, by implication, eating behaviour in infancy may predispose to later obesity(Reference Carnell and Wardle9, Reference Taveras, Scanlon and Birch10). It has also been proposed that an infant's temperamental style and how parents react to it may relate to later obesity(Reference Paul, Bartok and Downs1). Studies have found that infants with a so-called ‘difficult’ temperament (difficult to sooth; distress to limitations) show increased weight gain(Reference Carey11, Reference Darlington and Wright12) possibly because food is used to sooth, resulting in a higher energy intake(Reference Carey11). It is thus hypothesised that infants with a ‘difficult’ temperament may be more likely to go on to become obese.
The Gateshead Millennium Study cohort
The Gateshead Millennium Study (GMS) birth cohort was established to examine infant eating behaviour prospectively and relate this to subsequent growth and weight gain and has been described in a number of publications including a recent cohort profile(Reference Parkinson, Pearce and Dale13). The study aimed at recruiting all babies born to Gateshead resident mothers in pre-specified recruiting weeks between June 1999 and May 2000. Mothers were approached in the maternity unit or within the first week. We successfully recruited 1011 mothers of 1029 babies, 81% of those eligible. Baseline socio-demographic data were collected at the first interview and this was followed by four postal questionnaires in the first year (6 weeks, 4, 8 and 12 months) and a health check at age 13 months, where research nurses measured weight and length and collected parental heights and weights also. There was a further questionnaire at age 2½ years and once the children reached school age, the cohort was re-traced and funding obtained to study the children in school and at home between the ages of 7 and 8 years. Ethical approval was obtained at each stage.
The aim of this paper is to review all the relevant GMS findings to look specifically at the relationship between feeding transitions (stopping breast-feeding, starting solids) and infancy weight gain and eating behaviour, and describe new analyses relating infant eating behaviour and temperamental characteristics to infancy weight gain and childhood adiposity at age 6–8 years.
Published findings to date
The cohort was representative of an urban British population(Reference Parkinson, Pearce and Dale13) apart from their ethnic makeup, as the only significant minority group (3%) were members of an ultra orthodox Jewish group(Reference Wright, Stone and Parkinson14). At the time of recruitment, the cohort had good representation from across the socio-economic range, with 24% infants living in unwaged households and 71% of mothers having left school at 16.
Infant feeding
Just over half of the cohort commenced breast-feeding, but this had fallen to less than a third by 6 weeks(Reference Wright, Parkinson and Scott15). This meant that we could look at how these different feeding groups differed in weight gain. We found that the 20% breast-fed for longest (over 4 months) showed the slowest weight gain over the first year (after adjusting for birth weight) and the lowest BMI at age 13 months. However, these children were also the shortest after adjusting for parental height(Reference Wright, Parkinson and Scott15). While this might suggest to some that longer breast-feeding was actually associated with slower growth, we also noted that the group with the most rapid growth and weight gain and highest BMI were those infants who started breast-feeding, but stopped within 6 weeks, rather than those bottle-fed from birth. The probit study of breast-feeding promotion similarly found the slowest growth and weight gain in those with the longest breast-feeding duration when all their subjects were stratified simply by breast-feeding duration, but in contrast, when examined per randomisation, children randomised to the intervention arm, who had greater overall exposure to breast-feeding, showed rather more rapid growth on average than infants in the control arm(Reference Kramer, Guo and Platt16). The authors proposed that the likely explanation for the former finding was reverse causation: that is, the heavier, taller babies were more demanding and thus their mothers were more likely to stop breast-feeding. In our cohort, this was further supported by the observation that the commonest reason cited by mothers for giving up breast-feeding early was their babies' excessive hunger and feeding frequency(Reference Wright, Parkinson and Scott15).
The pattern of complementary feeding was fairly typical for a UK cohort at that time (1999–2000), before the UK adopted the WHO recommendation that solids feeding should be deferred until 6 months(Reference Hamlyn, Brooker and Oleinikova17); only one in five had started solids by the age of 3 months, but 95% had done so by 4 months(Reference Wright, Parkinson and Drewett18). The two commonest reasons the mothers gave for starting solids were that their baby was hungry and that the time ‘seemed right’. The mothers of those weaned earliest (before 3 months) were most likely to cite hunger as a reason(Reference Wright, Parkinson and Drewett18). Because of the large number of infancy weights available (an average of eleven per infant), we were able to examine how weight gain at different stages in the first year related to the age solids were started. On average the heavier the infant from birth onwards, the earlier solids were started; however, weight gain from birth to 6 weeks was the strongest predictor of age of first solids, while weight gain from 6 weeks to a year showed no association(Reference Wright, Parkinson and Drewett18).
We would argue that these data all work together to provide a rather different picture from the conventional idea that bottle feeding and early solids are simply causal risk factors for later obesity. While an infant's weight at 12 months may be correlated with age at first solids or duration of breast-feeding, we have to be aware that this may simply reflect the fact that this infant was heavier and hungrier at an earlier age and thus that the child's early behaviour determined the timing of these transitions (Fig. 1).

Fig. 1. A schematic illustration of the possible role of reverse causation with respect to infant feeding and growth in infancy.
This is not to say that prolonging breast-feeding and delaying solids are unimportant. Children in the WHO growth chart cohort who were drawn from six centres worldwide and all exclusively breast-fed up to 5–6 months showed the same growth in length, but slower weight gain than UK children(Reference Wright, Lakshman and Emmett19), which would suggest that longer breast-feeding may be relatively protective against later overweight. Deferring solids is also associated with longer durations of breast-feeding(Reference Wright, Parkinson and Scott15, Reference Fisk, Crozier and Inskip20), higher frequency(Reference Hornell, Hofvander and Kylberg21) and larger volumes taken(Reference Cohen, Brown and Canahuati22), which confers many other important health benefits. However, systematic reviews have now demonstrated that breast-feeding shows only weak associations with later obesity after full adjustment for confounding(Reference Owen, Martin and Whincup23, Reference Owen, Martin and Whincup24) so it should be recognised that preventive interventions targeting infant feeding are likely to be a good deal less effective for the prevention of obesity than retrospective studies would suggest.
Appetite and weight gain
What the described results do illustrate is that all babies influence their world and are not just passive empty vessels into which parents pour milk and spoon food. However, remarkably little is known about eating behaviour in infancy and even less about how it tracks onto later adiposity or eating behaviour, partly because of a lack of measures of eating behaviour validated for this age range. The initial focus of the GMS was to study weight faltering and thus the central purpose and the most novel aspect of this cohort study were to record infant feeding and eating behaviours prospectively through infancy and beyond. We asked a wide range of questions drawn from previous studies, surveys(Reference Foster, Laider and Cheesbrough25) and clinical experience and repeated them at different time points (3 days, 6 weeks, 4, 8 and 12 months). We then grouped certain questions into scores based on our prior ideas about causes of slow weight gain. Thus, we developed scores of maternal feeding anxiety, about the child's tendency to avoid being fed (avoidant behaviour) and the mother's response to food refusal (Reference Wright, Parkinson and Drewett26). At each time point, parents were asked to rate their child's appetite on a five-point scale (Fig. 2). Appetite was a significant predictor of weight gain at every age and the ratings at both 6 weeks and 12 months were independently associated with weight gain to 12 months(Reference Wright, Parkinson and Drewett26). Avoidant behaviour and maternal feeding anxiety were associated with weight gain, but not after adjustment for appetite. In contrast, maternal response to food refusal was the strongest predictor of low weight gain at 12 months, even after adjustment for appetite, leading to the suggestion that maternal over persuasion could actually inadvertently suppress intake(Reference Wright, Parkinson and Drewett26).

Fig. 2. Frequency distribution of parental ratings of infant appetite (percentage in each category) at ages 6 weeks and 12 months in Gateshead Millennium cohort.
While appetite was consistently associated with weight gain at the same age, this was a single variable, and half of all children rated as being in the top appetite category (see Fig. 2). This was thus an undiscriminating measure for the high appetites that might predict later obesity. The word ‘appetite’ also appeared to have a different meaning for parents at different stages in the first year(Reference Wright, Parkinson and Drewett26) and we have now shown that infancy appetite ratings were unrelated to BMI by age 7–8(Reference Parkinson, Drewett and Le Couteur27). Thus, before we began analysing data collected in childhood, we embarked on a further analysis to consider the extent to which eating behaviour and temperamental characteristics in infancy predicts infancy weight gain and to use these results to develop an infancy eating score that could be related to adiposity and eating behaviour later in childhood.
Method
Data collected in infancy
Anthropometric
Routinely collected clinic weights were returned with questionnaires by parents throughout the first year. At age 13 months, children were seen by research nurses who measured length using the Raven rollameter and weight using Seca scales.
Development of infancy eating avidity score
The parent report questionnaires used in this study included a wide range of other questions selected to describe hunger, eating vigour and enthusiasm for food. Many of these correlated significantly with both weight gain and appetite in the first year. We thus set out to investigate whether it was possible to use multiple variables to develop an infancy eating avidity score (IEAS) that would more effectively discriminate children at both extremes of the feeding spectrum.
The parent report questionnaire at 12 months included twenty-five questions drawn from previous research and clinical practice selected to describe enthusiasm and appetite or, conversely, aversion to solid food as well as any oro-motor feeding difficulties. Working in SPSS v15 all these variables were first explored individually and then collectively, using principal components analysis with the aim of identifying domains of eating behaviour. This revealed four apparently coherent components: avidity (18% variance), avoidance (11%), stress (9%) and physical/medical factors (8%). However, when the four components were used to construct factor scores per child, none of these four scores showed a significant association with either weight gain in the first year or with BMI aged 4 years.
A new statistical approach to the construction of an avidity score was then taken. This used general linear regression modelling to identify which variables independently predicted conditional weight gain (CWG) from birth to 1 year. The twelve variables that showed a borderline univariable association with CWG (P⩽0·2) were added to a multivariable general linear model. Variables making little contribution were then removed, leaving six variables that were independently predictive (P⩽0·05) (Table 1). These items were then combined by summing each regression coefficient, multiplied by the infant's observed response to the corresponding item, to produce each child's IEAS at age 1 year. This score was also divided into categories (high, average and low) by using natural break points in the distribution (Fig. 3).

Fig. 3. Infant Avidity Eating Score distribution in Gateshead Millennium cohort, showing natural break points for categories of low, average and high eating avidity.
Table 1. Variables independently associated with conditional weight gain (CWG) 0–12 months in general linear regression modelFootnote *
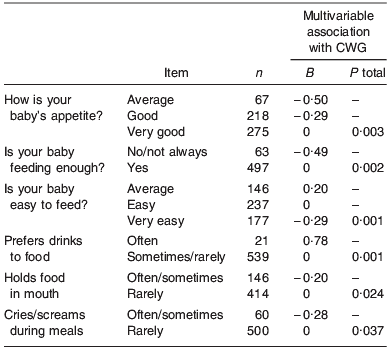
* All variables in table were entered into the general linear regression model simultaneously and the B and P values for each variable are thus adjusted for all other variables in the model.
Temperament
Mothers completed the Infant Behaviour Questionnaire at 6 weeks and 8 months. The Infant Behaviour Questionnaire consists of ninety items relating to infant behaviour during the previous week(Reference Rothbart28). At 6 weeks thirty-eight items were not included in the questionnaire because they were not relevant for that age group. A further four questions from the 6-week questionnaire and five questions from the 8-month questionnaire were excluded from the analysis because 15% or more of the respondents indicated the question was irrelevant(Reference Worobey29). The remaining questions were used to derive a score for five temperament dimensions at 6 weeks and 8 months: activity level, distress to limitations (persistence), smiling and laughter (‘smiliness’), distress and latency to approach sudden or novel stimuli (fear) and ‘soothability’. A further score for duration to orienting (attention) was derived at 8 months. Parents responded to the Infant Behaviour Questionnaire items on a seven-point scale from never to always. The questions were coded such that seven indicated the greatest activity, persistence, ‘smiliness’, etc. (e.g. an ‘always’ response to ‘During sleep how often does your baby toss in the cot?’) and one indicated the least (e.g. an ‘always’ response to ‘During feeding how often does your baby lie or sit quietly?’). As recommended by the original authors the total score for all relevant questions was divided by the number of relevant questions answered in that dimension, to give an average score for each of the temperament dimensions.
Data collected in childhood
The Child Eating Behaviour Questionnaire(Reference Wardle, Guthrie and Sanderson30) and the Child Feeding Questionnaire(Reference Birch, Fisher and Grimm-Thomas31) were completed by parents when the child was aged 6–8 years. At age 7–8 years, children were visited at school where research staff measured height with a Leicester portable measure and weight and leg-to-leg bioelectrical impedance with the Tanita TBF-300MA. Measurements were also taken of triceps and subscapular skinfolds, using Holtain skinfold callipers and waist circumference using a non-stretchable tape measure.
Analysis and power
All heights, lengths and weights were converted into Z-scores compared to the UK 1990 reference(Reference Freeman, Cole and Chinn32). Change in weight Z-score from birth to 12 months conditional on birth weight(Reference Wright, Parkinson and Drewett33) was calculated to give a figure for infancy CWG. Waist and skinfolds were also converted into Z-scores using the best available external references(Reference McCarthy, Jarrett and Crawley34, Reference Tanner and Whitehouse35) and the mean skinfolds Z-score taken. The bioelectrical impedance data were converted into Z-scores for fat and lean, standardised for height, gender and age, using reference data from the Avon Longitudinal Study of Parents and Children cohort(Reference Sherriff, Wright and Reilly36). Anthropometry, skinfolds and bioelectrical impedance were then combined using factor analysis, to create an adiposity index(Reference Wright, Cox and Sherriff37).
Post hoc analysis would suggest that with approximately 500 subjects the minimum detectable correlation would be about 0·18. This number would give 80% power to detect a statistically significant relative risk of 2·2 for high adiposity between children with IEAS in the top third compared to the remainder.
Exclusions
Analyses at 1 year excluded preterm infants as well as thirty-three Haredi-Jewish and eight Muslim infants as they all had very different growth and feeding patterns in the first year(Reference Wright, Stone and Parkinson14). Only seven Haredi or Muslim children had follow-up data at age 7 years so they were excluded for all analyses.
Results
Infancy eating avidity score as a predictor of growth and adiposity
The IEAS was available for 561 eligible children who also had weight data at a year and explained 8% of the variability in CWG from 0 to 12 months. There was substantial clustering of scores in the centre of the distribution, with 219 (37%) children having the median value, but then a much wider spread in both directions; 33% of children with values below the median were categorised as having a low IEAS and 26% above the median as having a high score (Fig. 3).
The growth characteristics of the cohort members in infancy and at follow up are described elsewhere(Reference Wright, Cox and Sherriff37). Growth of children with IEAS data did not differ from the cohort as a whole. At age 7–8 years, although correlation coefficients were low, IEAS was significantly correlated with both height and BMI, but an apparent correlation with waist and skinfolds became non-significant after adjustment for height(Reference Wright, Cox and Sherriff37). Children with high IEAS in infancy showed a consistent but non-significant tendency to be in the overfat range (above 90th centile) for the adiposity index at 7 years(Reference Wright, Cox and Sherriff37). There was no association between the factor scores generated from the initial principal components analysis and later adiposity.
Since the IEAS is a measure of feeding behaviours selected specifically because of their association with infancy weight gain, we also examined the overall association between infancy CWG and the same outcome measures. This revealed that CWG generally showed a stronger association with measures of height, BMI and lean mass than with adiposity at age 7 years(Reference Wright, Cox and Sherriff37).
Infancy eating avidity score and later eating behaviour
The IEAS significantly correlated with two of the seven Child Eating Behaviour Questionnaire domains (satiety responsiveness and food fussiness) collected at 6–8 years, but with only one of the seven Child Feeding Questionnaire domains (perceived child overweight) collected at age 6–8 years (Tables 2 and 3). At the age 6–8 years a child with a high IEAS has low satiety responsiveness, low food fussiness and their parents were more likely to perceive their child as overweight. Thus, the IEAS did predict a later aspect of eating behaviour that is thought to relate closely to obesity (poor satiety responsiveness) and also predicted parental worries about their child being overweight. However, a large number of statistical tests were carried out with relatively few showing statistical significance, so we cannot rule out the possibility that these are chance findings.
Table 2. Correlation between the eating score at 12 months and domain scores from the Child Eating Behaviour Questionnaire (CEBQ) and the Child Feeding Questionnaire (CFQ) taken at child's age 6–8 years

ρ, Spearman correlation coefficient.
Table 3. Correlation of temperament domains at age 6 weeks and 8 months with weight gain and Infant Eating Avidity Score (IEAS)

CWG, conditional weight gain 0–12 months; ρ, Spearman correlation coefficient.
Temperament
Parents of 831 (80·7%) subjects completed the 6-week questionnaire and 676 (65·7%) the 8-month questionnaire. There were only weak to moderate correlations between corresponding domains rated at both 6 weeks and 8 months (correlation coefficients activity 0·34, persistence 0·32, smiliness 0·30, fear 0·11; P<0·001 for all). There were no correlations greater than R=0·1 between either CWG or IEAS with any of the temperament domains at either 6 weeks or 8 months (Table 3). Only one, soothability at 6 weeks, achieved marginal statistical significance. No association was found between any of the domains and later adiposity (data not shown).
Discussion
From this review of a wide range of data from the GMS cohort, a picture emerges of infancy as a period where all children are hungry, reflecting the energy requirement of rapid growth and acquisition of fat stores characteristic of this period. Those who are hungriest seem as likely to be growing into tall children as becoming obese and we have not been able to identify any behavioural or temperamental characteristics that distinguish the two.
Infancy eating avidity score and adiposity
These results illustrate the difficulty of constructing an eating score in the first year of life. All parental ratings are subjective and are likely to vary with parental understanding of the meaning of the questions, as well as their perception of their child's behaviour. We therefore set out to develop a measure of infant eating avidity that was not reliant on one single subjective rating, such as appetite alone. There were a number of candidate behavioural questions, selected from the original questionnaires because they appeared to describe enthusiasm for, or avoidance of, eating. These grouped together coherently in a factor analysis, but the resulting factors were found to be unrelated to weight gain in infancy or adiposity in childhood. When a more empirical analytic technique was adopted, only six of these questions remained independently associated with weight gain at 1 year. This suggests that many descriptions of eating, even if they have face validity, may not be helpful in assessing actual intake. This would be in keeping with clinical experience where parental claims about feeding are often not reflected in their child's growth pattern.
So what are the likely meanings of the questions that proved predictive of weight gain? The rating of appetite and ‘whether baby feeds enough’ seem to relate to overall intake and were positively associated with infancy weight gain. ‘Holds food in mouth’ and ‘cries during feeds’ are both behaviours usually associated with feeding aversion and were inversely related to infancy weight gain, as would be expected. ‘Prefers drink to food’, however, was related to higher weight gain, possibly because this variable captures children who drink large volumes of milk. Finally, the ‘easy to feed’ variable was related to lower weight gain, but only when adjusted for the other variables in the IEAS. One could speculate that this might be explained by ‘very easy’ babies becoming relatively underfed because of their undemanding nature, but clearly further work would be needed to explore this.
The questions had originally been compiled mainly for the detection of weight faltering rather than overeating, but when combined to form the IEAS the measure scaled reasonably across the whole range of weight gain in infancy and explained more than four times the variance explained by the single appetite variable. It is not yet clear whether the combination of the six IEAS items might be useful in other populations and the limits of the age range where the IEAS effectively measures avidity need to be defined. Further work is now needed to test the validity of the IEAS in other groups, particularly ones with a higher risk of obesity, such as the children of obese mothers.
Recent work in this area has suggested that childhood eating behaviours associated with adiposity are quite strongly heritable(Reference Wardle and Carnell38) and thus one might expect that there would be behavioural associations with adiposity present from birth. However, it has also been shown that the degree of heritability increases with the age of the children studied(Reference Haworth, Carnell and Meaburn39), suggesting that a heritable tendency to overeat may emerge after infancy, once children are exposed to both the family and the wider food environment. It must also be remembered, however, that this study was not powered to detect effects in small subgroups, so it is quite possible that there could be meaningful associations for the most avid eaters that could only be identified by a larger study.
Temperament
Temperament scales were designed to measure intrinsic, biologically driven behavioural characteristics in the child, which the literature suggested might relate to infancy weight gain. However, it is not clear whether this scale really was measuring a stable characteristic, as we found only weak to moderate correlation between the 6 weeks and 8 months assessments. This is generally in keeping with the published literature, though one small recent study suggested that temperament was stable between age 3 and 9 months, but drew this conclusion after testing just two domains, with correlations between 0·25 and 0·6(Reference Slining, Adair and Goldman40). The questionnaire we used (Rothbart) was calibrated for infants aged 3 months and the author of the scale in fact argues that an infant's temperament is not stable until 6 months(Reference Rothbart, Derryberry and Hershey41). The infants in this study were rated initially at 6–8 weeks, at a stage when some infant problems such as colic, may distort early ratings of intensity and mood.
Another problem facing temperament research is the use of different scales with different domains and it is not always clear how these different scales map onto each other. Thomas, Chess and Birch, researchers of temperament starting from the 1960s, defined a ‘difficult’ temperament as: ‘frequent negative affect; irregularity in sleeping, eating and eliminating; intense reactions to stimuli; initial aversion and slow adaption to changes in the environment’(Reference Thomas, Chess and Birch42). Carey argues that a ‘non-difficult’ child should be active(Reference Carey and McDevitt43), whereas in a recent cohort study the outcome of high activity (measured by Rothbart's scale) was included among other domains such as low effortful control and high negative affectivity in describing difficult temperaments for children aged 2 years(Reference Pesonen, Raikkonen and Lano44).
A ‘difficult’ temperament in infancy is often referred to in relation to later overweight, but our study, one of the largest studies to measure temperament in infancy, found no links between temperamental domain scores and any weight outcomes in infancy or in later childhood. Three studies in the past 20 years have found an association between measures of a ‘difficult’ temperament and rapid weight gain in infancy(Reference Carey11, Reference Darlington and Wright12, Reference Niegel, Ystrom and Vollrath45), while two further studies have found ‘difficult’ temperament domains in infancy to be related to other weight outcomes (BMI and skinfolds), either in infancy(Reference Slining, Adair and Goldman40) or later in childhood(Reference Wu, Dixon and Dalton46). However, one of these, a large cohort study, showed no association between difficult temperament in infancy and being overweight and only a weak association with weight gain in infancy(Reference Niegel, Ystrom and Vollrath45).
The disparity between different studies is not surprising, since the large number of domains in each scale require multiple significance tests, making one or more significant results likely purely due to chance. With the known bias towards publishing positive results this makes it likely that plausible positive findings are published, while null findings are not. For example, in our dataset ‘soothability’ at 6 weeks was a borderline significant predictor of weight gain and in a small study with only limited data this could have been highlighted as a plausible association. In this instance, though, we were able to show that ‘soothability’ showed no correlation with any Child Eating Behaviour Questionnaire domains (reported by parents at age 6–8 years) or any other feeding variables and was just one of twenty-two different comparisons presented in that table alone, so it seems likely that the association of CWG with soothability was simply a chance finding.
In summary, we have not been able to replicate the findings of smaller studies suggesting associations of temperament with weight gain. In addition, we have found little evidence of stability in temperamental characteristics across infancy. This raises the possibility that current measures of temperament in infancy do not in fact capture any enduring behavioural/biological characteristics. We recommend that researchers should be cautious when considering the notion of temperament and should publish negative as well as positive findings.
Conclusions
While infancy initially appears to be a logical place to identify children at risk of future obesity and prevent its onset, the collective findings from this substantial population-based study largely suggest otherwise. Previously observed associations between feeding patterns in infancy and later obesity, when examined prospectively, are revealed to be the result of reverse causation. Associations with temperament could not be replicated and seem likely to reflect chance findings and perhaps publication bias. Even simply gaining weight rapidly in infancy predicts tall stature more than adiposity 7 years later, while infancy eating behaviour seems to relate only weakly to later adiposity. If infants destined to be obese do not have distinctively different eating behaviour or growth, it will be difficult to identify those for whom an early intervention might be of benefit and attempting this risks mislabelling infants destined to be tall rather than fat. Encouraging healthy milk and solid feeding practices in infancy makes sense for many reasons, as does setting more realistic and healthy standards of comparison, but the impact of initiatives in infancy are likely to have at best a marginal impact on adult obesity. In summary, having worked back ever earlier in search of a period when the onset of obesity can be prevented, we must now consider that there may be no such single period. The propensity to adult-type obesity seems to emerge progressively through childhood and young adulthood. If so, interventions more proximal to the outcome are likely to be much more fruitful.
Acknowledgements
Thanks are especially due to the GMS families and children for their participation in the study and to the research staff for their efforts. We are grateful to Ashley Adamson, who planned and ran the childhood phase of the study, and for her very helpful comments on the draft, to Andrea Sherriff and Mark Pearce for their statistical input, to Anna Boyce for her literature review and to the rest of the GMS study team (Anne Dale, Robert Drewett, Paul McArdle, Kathryn Parkinson and John Reilly) for their work on the study. We appreciate the practical support of Gateshead Health NHS Foundation Trust, Gateshead Education Authority and local schools and the support of an External Reference Group.
GMS study team: C.M.W. ran the infancy phase and helped plan the childhood phases of the study, participated in the analysis and drafted the paper. A.L.C. ran the parent questionnaire phase at age 5–6 years and helped plan the childhood phase of the study. K.M.C. undertook the main analyses and helped draft the paper. All authors have seen and commented on the manuscript. The authors declare no conflict of interest.
The GMS was supported by a grant from the National Prevention Research Initiative (incorporating funding from British Heart Foundation; Cancer Research UK; Department of Health; Diabetes UK; Economic and Social Research Council; Food Standards Agency; Medical Research Council; Research and Development Office for the Northern Ireland Health and Social Services; Chief Scientist Office, Scottish Government Health Directorates; Welsh Assembly Government and World Cancer Research Fund) and this analysis was undertaken with funding from the Chief Scientist Office, Scottish Government UK.
The cohort was first established with funding from the Henry Smith Charity and Sport Aiding Research in Kids and followed up with grants from Gateshead NHS Trust R&D, Northern and Yorkshire NHS R&D, and Northumberland, Tyne and Wear NHS Trust.










