INTRODUCTION
The introduction and spread of livestock viruses into the European Union (EU) has substantial veterinary and economic consequences and, in some cases, could impact on human health. Many viruses which infect livestock and humans are transmitted by arthropod vectors such as biting midges, mosquitoes, ticks and sand-flies. Vector-borne livestock viruses, which infect humans include Crimean-Congo haemorrhagic fever virus (CCHFV), Rift Valley fever virus (RVFV) and West Nile virus (WNV). CCHFV, for example, is endemic in parts of eastern Europe and may be transmitted from human-to-human nosocomially and from infected livestock meat to humans [Reference Vorou, Pierroutsakos and Maltezou1], with higher incidence in abattoir workers and butchers in Iran [Reference Fakoorziba2]. Climate change will impact not only on the distribution and abundance of vectors but also on the interaction between the virus and its vector [Reference Baylis and Githeko3, Reference Gould and Higgs4]. Recently, for example, bluetongue virus (BTV) serotype 8 has emerged in north-western Europe including the UK as a result of higher temperatures facilitating transmission of the virus by indigenous midge vectors of the Culicoides obsoletus complex [Reference Mehlhorn5, Reference Landeg6]. Since 1998, incursions of BTV in southern Europe have occurred due to the northwards expansion in range of the traditional vector, Culicoides imicola [Reference Purse7]. The recent emergence of BTV in livestock in northern Europe highlights the need to understand the potential effects of climate change on the occurrence, distribution and prevalence of livestock diseases. Although the potential problems associated with climate change are now becoming widely recognized, quantitative data on the distribution and abundance of many vector species within the EU are not available and the influence of climate change on the distribution of the vectors is highly uncertain. In addition to the vector route, there are other routes of transmission for viruses to livestock in EU member states, including international trade in livestock, importation of meat products and companion animals, and exposure to wildlife. Transmission of viruses through some routes will be more susceptible to the effects of climate change than other routes.
It is important to consider climate change in combination with other factors [Reference Gould and Higgs4, Reference Gale8]. Host ecology, host behaviour and increased globalization including the transportation both of people and cargo containers are important [Reference Reiter9]. Recently, transmission of chikungunya virus (CHIKV) was reported for the first time outside the tropics with the emergence of cases in southern Europe. Climate change may not necessarily have been the major factor [Reference Gould and Higgs4, Reference Reiter9]. Thus, the virus was imported to a town in northern Italy by a traveller returning from India, while human activities had altered the local ecology allowing establishment of the mosquito vector, Aedes albopictus. The eggs of the mosquito may have been introduced in cargo containers containing shipments of loose tyres imported from Asia [Reference Reiter9]. The ability of the virus itself to respond to change is an important factor. Thus, for example, a single mutation in CHIKV has been identified as promoting infection in the mosquito A. albopictus over the recognized vector, Aedes aegypti [Reference Tsetsarkin10]. This mutation increases the potential for CHIKV to permanently extend its range into Europe and the Americas, where A. albopictus has established over the last 20 years [Reference Medlock11].
The objective of this study was to prioritize five vector-borne viruses according to the risk of incursion into the EU and the risk of becoming endemic within the EU, both at the present time and after the impact of climate change assumed to have occurred in the 2080s. In addition, the impact of climate change was broken down into the various routes of introduction. A qualitative risk-assessment approach based on elicitation of expert opinion was used. The impetus of this work was to facilitate the identification of appropriate risk-management options in relation to adaptations to climate change. The viruses studied were African horse sickness virus (AHSV), CCHFV, RVFV, African swine fever virus (ASFV) and WNV. AHSV is transmitted by Culicoides midges, ASFV and CCHFV by ticks, and RVFV and WNV by mosquitoes. The 2080s was selected as the decade to consider for the impact of climate change as previous studies [12–Reference Christensen and Christensen14] have generated predictions for the 2080s period or ‘by the end of the 21st century’.
METHODS
A qualitative risk assessment for five vector-borne viruses listed as notifiable by the World Organisation for Animal Health [15], was conducted based on the Office International des Epizooties (OIE) framework [16] and therefore included an assessment of the probabilities of release, exposure and consequence. The risk pathway is presented in Figure 1 and the full definitions of the release, exposure and consequence assessments are set out by OIE [16]. In this context, release corresponds to the likelihood of entry of the virus into the EU, exposure considers the likelihood of susceptible animals becoming exposed to the virus, and consequence defines the likelihood of biological, environmental or economic consequences, and their likely magnitude. Exposure and consequence are conditional on release and exposure, respectively.
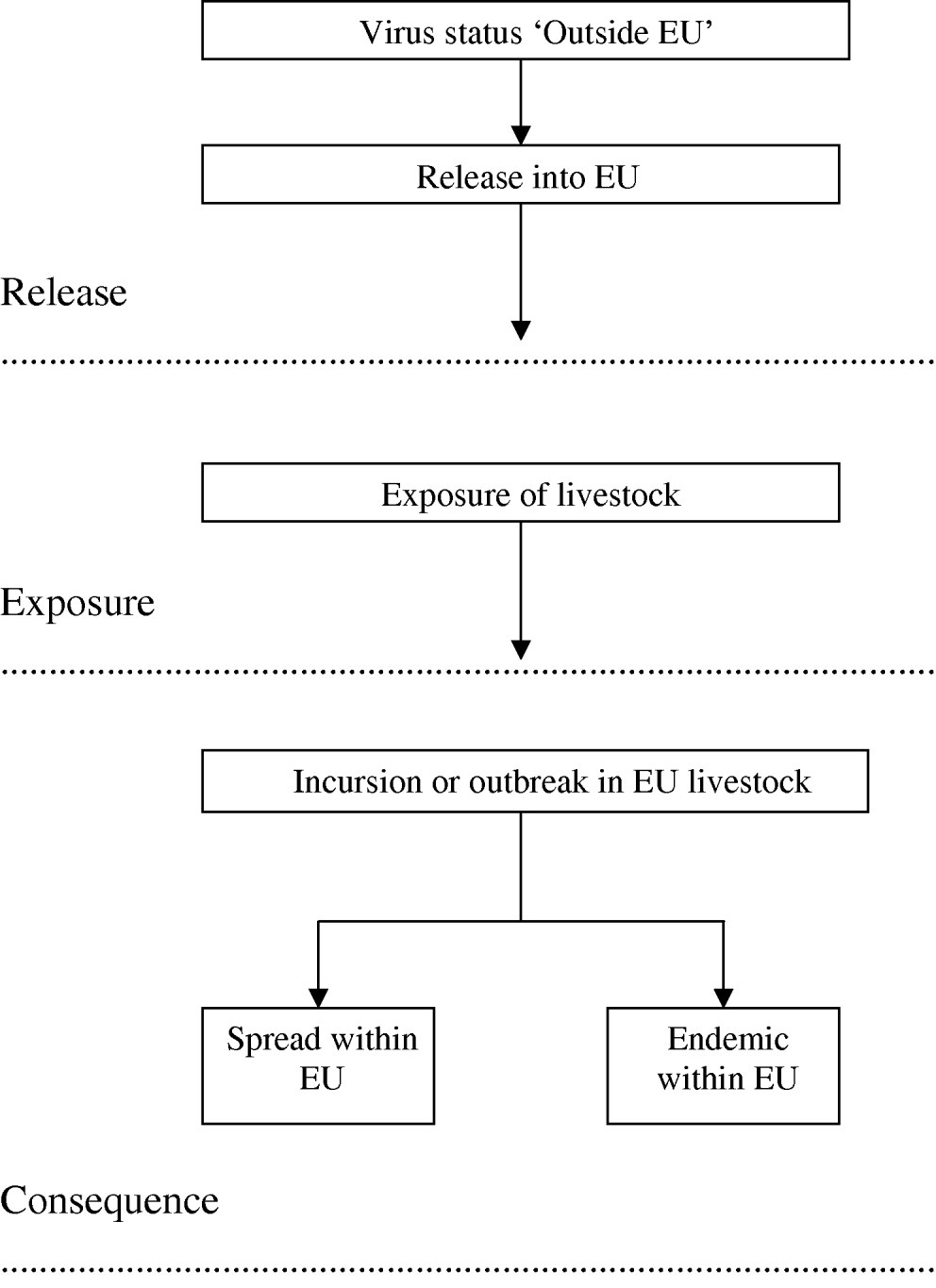
Fig. 1. Risk pathway for the risk of vector-borne livestock disease incursion, spread and becoming endemic in the EU.
Elicitation of data through expert opinion methodology
These data were qualitative estimates of the probabilities of release, exposure and consequence and were obtained by elicitation of expert opinion through questionnaires using the modified-Delphi technique [Reference Ayyub17]. The approach was similar to that used previously for estimating the risk of importation of foot-and-mouth disease into Europe [Reference Gallagher18]. A workshop enabled the ‘conditioning’ of the experts prior to their completing the questionnaire for a second time and facilitated clarification of any interpretation issues with the questionnaire.
Structure of the questionnaire and accompanying information
Each virus-specific questionnaire was divided into five parts. Part 1 asked for background information on the expert. Throughout the remainder of the questionnaire, the expert was asked to answer questions relating to the virus both currently, and predicted in the 2080s after climate change. In the ‘top level’ analysis, the experts were asked to assess the risk through all routes combined, while further questions within each part of the questionnaire addressed the probabilities of release, exposure and outbreak through each of six routes, namely infected vector, infected livestock, meat or meat products from infected animals, infected wild animals, infected pets and infected persons. Part 2 asked the experts to indicate the current and future (2080) risk of incursions/outbreaks of the virus in parts of the world outside the EU to provide an indication of the worldwide prevalence. Part 3 elicited expert opinion on the effect of climate change on the risk of release into the EU, given the current and predicted worldwide prevalence in Part 2. In Part 4, the experts were asked to assess the effect of climate change on the risk of exposure to livestock within the EU, given that the virus has entered the EU through any of the routes in Part 3. The experts were asked to consider the effect that climate change may have on farming practices with respect to changes in intensity of farming and movement of animals, when making their judgement. In Part 5, expert opinion was elicited on the effect of climate change on the consequences. Three consequences were considered, namely risk of incursion (or outbreak) given exposure, risk of spread within the EU given incursion, and risk of becoming endemic in the EU given incursion (Fig. 1). Here, only the results for the risk of incursion and risk of becoming endemic are provided. Each expert completed the same questionnaire twice; the first prior to the workshop and the second during the second half of the workshop. Included with the first questionnaire was supportive literature on definitions and climate change scenarios. The climate change scenarios across Europe in the 2080s were described broadly in the documentation as higher temperatures particularly in southern Europe, increasing frequency of hot summers, a wetter northern Europe, a drier southern Europe and more extreme weather conditions with increased risks of heat wave, drought and flooding [12, Reference Christensen and Christensen14, Reference Beniston and Diaz19].
The expert opinion workshop
The results of the first round of questionnaires were presented to the experts at the workshop. A presentation on climate change in Europe was given to standardize the experts' understanding of the climate predictions for the 2080s. In addition, breakout groups promoted discussion on each of the following topics: (1) the impact of climate change on farming; (2) the vertebrate reservoir hosts in Europe; and (3) the impact of climate change on the pathogen/vector interaction. At the end of the workshop, each expert repeated the questionnaire for each of the five viruses without reference to their answers from the first questionnaire. Only data from the second questionnaire were used in the risk assessment.
Selection and weighting of experts
Experts were chosen from across Europe on the basis of their expertise in nine vector-borne viruses listed by the World Organization for Animal Health [15], namely AHSV, RVFV, CCHFV, ASFV, WNV, Venezuelan equine encephalitis virus, Western equine encephalitis virus, Japanese encephalitis virus and vesicular stomatitis virus. For the purpose of the workshop, five of those viruses were selected, namely AHSV, RVFV, CCHFV, ASFV and WNV, on the basis of expertise of the experts given in the first round of questionnaires. In total, 18 experts were recruited. Of those, 16 completed the questionnaire individually, with two completing the questionnaire jointly. In total therefore, 17 questionnaires were completed for each virus. This was to enable a comparison at the group level and was judged to be an essential part of the process. It was also felt that although some experts may have considered their expertise to be very low for the virus per se, they may have information on the vector or the ecology, for example, or other factors which could be drawn upon. The questionnaire included a self-ranking of the expert's level of expertise for each virus (Table 1). To accommodate the differences between experts in their levels of expertise for each virus, each level of expertise was given a weighting (W i,j, where i=number of expert, i=1, …, 17, j=virus). For each virus, a scoring system was used that gave a score of 5 for an expert with ‘very high’ expertise decreasing to a score of 1 for an expert with a ‘very low’ level of expertise. Consequently for each virus, an overall risk was obtained for each expert and was combined with the risks from the other experts by allowing each expert to contribute W i,j risks. For example, if expert 3 has expertise level ‘medium’ for WNV and gave an overall assessment of ‘low’ then that expert will contribute 3 (W 3,WNV=3) ‘lows’ to the overall assessment. Similarly, expert 5, who has a ‘low’ expertise in WNV but estimated the risk to be ‘negligible’ will contribute two ‘negligibles’ (W 5,WNV=2). This method was applied across the experts to give a weighted distribution of the overall risk, from which a median value and 10th and 90th percentiles were derived. To investigate the impact of the weighting scheme on the results a sensitivity analysis was undertaken.
Table 1. Summary of the expertise of the experts for each virus
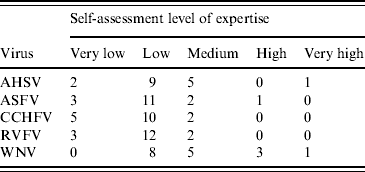
AHSV, African horse sickness virus; ASFV, African swine fever virus; CCHFV, Crimean-Congo haemorrhagic fever virus; RVFV, Rift Valley fever virus, WNV, West Nile virus.
Very high, Published many papers, led research projects on this virus, including an investigation of the impact of climate change.
High, Published many papers, led research projects on this virus.
Medium, Worked on research projects and contributed to papers on the virus.
Low, Some background knowledge, but no direct research experience on virus.
Very low, Minimal background knowledge of virus.
Qualitative assessment of risk
The definitions of the probabilities of an event occurring [Reference Moutou, Dufour and Ivanov20] were given to the experts in an accompanying information sheet as: negligible (so rare it does not merit consideration); low (rare but does occur); medium (occurs regularly); and high (occurs very regularly). Experts were asked to qualify the probabilities of release, exposure and consequence, given these definitions.
Due to the conditional nature of the exposure and consequence probabilities, the overall estimate of risk for a pathway (Fig. 1) can be derived by ‘multiplying’ the probabilities of release, exposure and consequence. A matrix was defined to determine the result of multiplying two qualitative probabilities (Table 2). The structure of the matrix accounts for the fact that probabilities are always between 0 and 1. Therefore, when ‘multiplying’ probabilities together the resulting probability must be, at the absolute maximum, equal to the lower probability. Other matrix approaches [Reference Moutou, Dufour and Ivanov20] were also considered. However, these were not deemed appropriate because they do not capture the multiplicative nature of the risks being assessed here. For each virus and for each expert, the risk associated with each consequence was calculated for the current time and with climate change as predicted in 2080.
Table 2. Matrix used for multiplication of two qualitative probabilities

Assessment and comparison of the risks through different routes of transmission
The questionnaire specified six routes of entry of virus, namely vectors, livestock, wildlife, meat and meat products, persons and pets. While transmission of ASFV occurs from direct pig-to-pig contact, and also through consumption of meat products from infected pigs, transmission to livestock for the other four viruses studied here is primarily dependent on a competent vector. Thus, in the absence of a mosquito vector, the risk of direct transmission of WNV, for example, from birds to horses would be negligible. It was therefore explained in the questionnaire that exposure through a given route encompasses both direct and indirect contact with the virus, given that the virus has entered the EU through that route. The questionnaire cited soil, air, water, food, direct contact with other animals and humans, contact with a vector or exposure through the mechanics of biting as examples.
Sensitivity analysis
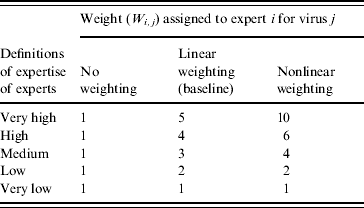
The method used for the weighting of experts can be considered subjective. We therefore considered another two systems: no weighting of experts and nonlinear weighting. The weights associated with these systems are presented in Table 3. Using the alternative weighting systems, the risk of incursion for AHSV, ASFV, CCHFV, RVFV and WNV in the EU were estimated and compared.
Table 3. Alternative systems used in the sensitivity analysis for the weighting of experts
RESULTS
The risks for two consequences, namely the risk of incursion and the risk of the virus becoming endemic in the EU at the ‘top level’ (i.e. through all routes combined) are compared under the current situation and with climate change. In addition, the risk of incursion is broken down into the individual routes of entry. All results refer to risks, which were linearly weighted (Table 3) to accommodate the level of expertise of the experts for each virus. Table 1 summarizes the expertise for the five viruses.
Risk of an incursion into the EU at the current time and with climate change
At the current time, the predicted risk of incursion in the EU from other parts of the world was greater for ASFV and WNV than for AHSV, CCHFV, and RVFV (Table 4). Climate change increased the predicted median risk for AHSV from ‘low’ to ‘medium’ while the median risks for the other viruses were unchanged (Table 4). This increase in risk for AHSV can be attributed to the experts' predicting an increase in the risk of both release and exposure (Fig. 1). For CCHFV and RVFV, climate change affected the 10th–90th percentiles, which is consistent with a small increase in predicted risk. This was due to an increase in the predicted risk of release and exposure for CCHFV and an increase in the risk of release (but not exposure) for RVFV with climate change (Fig. 1).
Table 4. Qualitative assessment of the risk of incursion of five vector-borne livestock viruses in the EU

AHSV, African horse sickness virus; ASFV, African swine fever virus; CCHFV, Crimean-Congo haemorrhagic fever virus; RVFV, Rift Valley fever virus, WNV, West Nile virus.
Risk of virus becoming endemic in the EU at the current time and with climate change
The current risk of becoming endemic was highest for WNV, as indicated by the median and lowest for RVFV, as indicated by the 10th–90th percentiles (Table 5). Climate change was predicted to have no impact on the predicted median risk for any of the five viruses becoming endemic in the EU. However, a predicted impact was apparent when considering the 10th–90th percentiles. In particular, the 10th–90th percentiles shifted for RVFV, and the 10th percentiles for AHSV and CCHFV increased, suggesting that climate change may, in the opinion of some experts, increase the risk of these viruses becoming endemic in the EU. Climate change did not appear to affect the predicted 10th–90th percentile risks for either ASFV or WNV becoming endemic in the EU. Further investigation revealed that the AHSV percentiles increased due to predicted increases in the probabilities of release, exposure and becoming endemic given incursion, and that the percentiles for CCHFV increased due to increases in the probabilities of release, exposure and becoming endemic given incursion (Fig. 1). The RVFV percentiles increased due to the increase in the probabilities of release and becoming endemic given incursion.
Table 5. Qualitative assessment of the risk of five vector-borne livestock viruses becoming endemic in the EU

AHSV, African horse sickness virus; ASFV, African swine fever virus; CCHFV, Crimean-Congo haemorrhagic fever virus; RVFV, Rift Valley fever virus, WNV, West Nile virus.
The ‘between-expert’ variation, as described by the 10th–90th percentiles, for the risk of becoming endemic for AHSV, CCHFV and WNV indicated a considerable degree of disagreement between experts (negligible–medium). Interestingly, there seemed to be greater agreement between experts for the prediction with climate change in that the 10th–90th percentile ranges for AHSV and CCHFV narrowed to low–medium. Agreement between experts was greater for RVFV and ASFV.
Impact of climate change on the routes of incursion into the EU
The risks of incursion into the EU from entry of virus through each of the six routes were estimated for each virus. The results given in Table 6 show those routes of entry for which the predicted risk of incursion was greater than negligible. The current median risk of incursion from entry of vectors was predicted to be non-negligible for each of the five viruses, as expected for vector-borne viruses. However, only for AHSV and CCHFV were the current risks of incursion from entry of infected vectors predicted to be highest for the six routes of entry studied. Thus for ASFV, the risks of incursion currently and for the 2080s from entry of meat and meat products and livestock were predicted to be higher than that through the entry of vectors. For WNV, the risk of incursion through entry of wildlife was predicted to be the highest currently. This reflects mosquitoes' feeding on infected migratory birds. For RVFV, the risk of incursion through entry of livestock was predicted to be higher currently than that through entry of vectors as judged by the 10th–90th percentiles.
Table 6. Qualitative assessment of the risk of incursion into the EU for five vector-borne virses: non-negligible routes of release
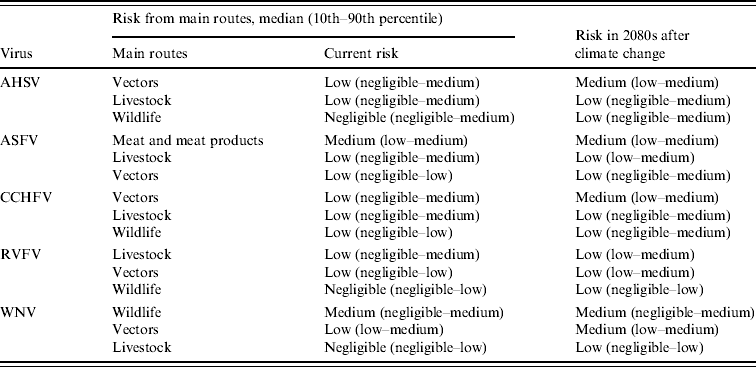
AHSV, African horse sickness virus; ASFV, African swine fever virus; CCHFV, Crimean-Congo haemorrhagic fever virus; RVFV, Rift Valley fever virus, WNV, West Nile virus.
Climate change was predicted to increase the median risk of incursion through entry of vectors from low to medium for AHSV, CCHFV and WNV (Table 6). For these three viruses, the changes in risk of incursion through entry of vectors were due to the experts believing that a change in the release and exposure probabilities would occur with climate change (Fig. 1), therefore suggesting that the vector range and/or vector density would increase. The predicted median risks of incursion through entry of vectors for ASFV and RVFV were not affected by climate change, but an increase was detected in the 90th percentile and 10th–90th percentile range, respectively, suggesting that the risk of these viruses through the vector route may also be increased by climate change.
It was predicted that climate change could increase the risks of incursion through entry of livestock for WNV, ASFV and RVFV to some degree. The predicted risk of ASFV through entry of meat and meat products from infected animals was not affected by climate change. Climate change was predicted to result in some increase in the risk of incursion through entry of wildlife for AHSV, RVFV and CCHFV, but not for WNV. This may reflect vectors' feeding on infected wildlife, either imported or entering the EU through migration.
Sensitivity analysis: risk of incursion
Re-analysing the expert opinion data using the additional expert weightings given in Table 3 (no weighting and nonlinear weighting) identified that the analysis was not very sensitive to the weights used. Comparing the risk of incursion (current climate) the only difference in the predicted median risks was for WNV. Thus the median was estimated to be medium for the no weighting and linear weighting, but was estimated to be low if using the nonlinear weighting. In addition, the 10th–90th percentile for ASFV was affected, being low–medium for no weighting and linear weighting, but negligible–medium for the nonlinear weighting. Under the 2080s climate change scenario, the only difference was for CCHFV with a medium estimate for the median risk being estimated when assuming the experts are equal in their expertise, but otherwise (linear and nonlinear weighting) the median was predicted to be low.
DISCUSSION
Investigations of climate changes during the last century suggest that the climate of Europe is changing at a rapid rate [Reference Gerstengarbe and Werner21] and this development will most likely continue during the next decades [22]. These climatic changes are particularly pronounced in central Europe. Climate change may impact on livestock diseases through its effect on a number of factors including the range and abundance of vectors and wildlife reservoirs, survival of pathogens in the environment, and farming practice [Reference Gale8]. These factors may interact with each other and also with social and anthropogenic changes, including habitat destruction and changes in land use, which occur both globally and locally, and increased mobility of people and movement of goods including livestock [Reference Reiter9].
In the case of BTV, incursion into southern Europe was supported by an expansion in range of its midge vector, C. imicola, from North Africa across the Mediterranean Sea through climate change [Reference Purse7]. Furthermore, higher temperatures increase the competence of the midge vector to transmit the virus between livestock [Reference Purse7, Reference Whitmann and Baylis23]. The importance of climate change in emergence of tick-borne viruses is less clear [Reference Randolph24], and other factors such as social, political and economic changes have been shown to be important for tick-borne encephalitis virus (TBEV) in humans in the Baltic states [Reference Sumilo25]. In several countries in Europe, the dramatic spike in TBEV cases in humans in 2006 may have reflected favourable weather conditions promoting outdoor recreational activities, rather than changes in tick abundance [Reference Randolph26]. For mosquito-borne viruses, there is also evidence from the WNV outbreak in Israel in 2000 that the magnitude of the minimum temperature during prolonged heat waves is the key climatic variable [Reference Paz27]. The 1999 outbreak in New York was preceded by a 3-month drought and a 2-week heat wave [Reference Rogers and Sanderson28].
The qualitative risk assessment developed here focused on the impact of climate change on the release, exposure and consequence for five vector-borne viruses, and was parameterized by elicitation of expert opinion. Issues such as the impact of climate change on the vector, host reservoir or characteristics and epidemiology of the pathogen, although implicit in the expert responses, were not specifically addressed. The information given to the experts on the predictions for climate change in Europe was broad and, furthermore, it was not possible to regionalize Europe in terms of climatic predictions for the 2080s. Experts were therefore asked to consider Europe as a whole, although it is well-known that regional differences in climate within Europe are important for tick-borne diseases. For example, TBEV foci in central Europe reflect areas where the climatic conditions allow the temporal synchrony required for tick-to-tick transmission, and predictions for the 2080s suggest that TBEV will be eliminated from central Europe due to breaking of the synchrony with the last foci remaining in Scandinavia in the 2080s [Reference Rogers and Randolph29].
The expertise of the experts differed for the five viruses with expertise across the group being greater for WNV and AHSV and lowest for CCHFV. However, it should be noted that the definition of ‘low’ expertise (Table 1) includes some background knowledge, albeit no direct research experience on the virus. In the case of CCHFV therefore, 12 of the 17 experts had some background knowledge or greater. The work presented here demonstrates the approach and preliminary results from a group of experts initially selected to cover nine vector-borne livestock viruses. For the purpose of the workshop, it was felt that five was the maximum number of viruses which could be covered, and these were selected based on the expertise of the experts. Future approaches could focus on selecting experts for just those five viruses. The sensitivity analysis showed that weighting of expertise had relatively little effect on the conclusions. This is probably due to the matrix used (Table 2). The overall risks of incursion into the EU of AHSV, RVFV and CCHFV from outside the EU were predicted to be increased by climate change while the risks for WNV and ASFV were predicted not to be affected (Table 4). Similar trends were predicted for the risks of becoming endemic in the EU (Table 5). The predicted risk of incursion through the entry of vectors was increased by climate change for all five viruses, the effect being strongest for AHSV, CCHFV and WNV according to the medians (Table 6).
In the case of AHSV, this prediction is not surprising and the effect of climate change on the risk from Culicoides-transmitted viruses to the UK's livestock industry has been known for some time [Reference Whitmann and Baylis23]. First, incursions of AHSV beyond its endemic areas (sub-Saharan Africa) have already occurred into the EU with outbreaks in Spain and Portugal [Reference Mellor and Hamblin30]. Second, the most important vector for AHSV, the biting midge C. imicola, has recently expanded its range northwards from northern Africa into southern Europe to include not only Portugal and Spain, but also Italy, Greece and even southern Switzerland. This is believed to be due to climate change [Reference Purse7]. Third, as with the related BTV, higher temperatures increase vector competence, such that novel midge species, including those abundant in northern Europe (e.g. the C. obsoletus complex), may be able to serve as vectors enabling AHSV to extend its range well beyond that of C. imicola [Reference Mehlhorn5, Reference Calvete31]. The distribution and spatial coincidence of C. imicola and the C. obsoletus group throughout the Iberian Peninsula has recently been modelled [Reference Calvete31].
Epidemics of CCHFV were first recorded in the Balkans in 1944 and in Africa in 1956 with outbreaks in Mauritania in 2003 [Reference Nabeth32] and Turkey in 2001–2008 [Reference Randolph and Ergonul33]. Although the virus has been isolated from several genera and species of Ixodid ticks, the main vectors involved in CCHFV transmission are ticks of the genus Hyalomma, particularly Hyalomma marginatum. For CCHFV, climate change is predicted to increase the risks of incursion through entry of both vectors and wildlife (Table 6). The wildlife reservoirs for CCHFV are small mammals, including hares, hedgehogs and rats [Reference Nalca, Whitehouse, Ergonil and Whitehouse34], which may benefit and increase in abundance through milder winters and enhanced heavy rainfall [Reference Carrara35]. It has been argued that migratory birds are not the reason for the sudden appearance of CCHFV in Turkey [Reference Randolph and Ergonul33]. Climate change and environmental changes may affect CCHFV epidemiology and trigger community outbreaks [Reference Vorou, Pierroutsakos and Maltezou1]. However, long-term trends in changing climate do not provide a sufficiently consistent explanation for the emergence of CCHFV in Turkey [Reference Randolph and Ergonul33]. Thus, it has been proposed that changes in human behaviour directly affecting the density of vertebrate host reservoirs are more important than climatic factors [Reference Randolph and Ergonul33]. In particular, war and terrorism in the region disrupted normal agricultural and hunting activities allowing weeds to grow and hare densities to increase. Although terrorist activities and war may have great impacts on the current and past emergences of CCHFV in Turkey and Crimea, climate change should not be neglected because certain environmental variables may affect the future dispersion and distribution of the Hyalomma vectors as well as that of the vertebrate reservoirs and livestock within Europe, and, furthermore, may affect the release of the virus from other parts of the world. It is possible that climate change will facilitate an expansion in the range of H. marginatum in Europe with creation of drier habitats. Both climate suitability for the Hyalomma tick vector and landscape fragmentation (through interspersion of agricultural land with shrub-type vegetation and forest) have been identified as important predictors of CCHFV cases in humans in Turkey [Reference Estrada-Peña36]. Fragmentation of the landscape and habitats may be affected by climate change in the future with decreasing rainfall increasing the grassland between forested areas.
The prediction that climate change has little impact on the overall risk of incursion of ASFV is consistent with the prediction of climate change having no effect on the risk through the main route which was identified as meat and meat products (Table 6). Climate change does increase the predicted risk of incursion of ASFV through the entry of vectors (increase in the 90th percentile), but since this is a minor route compared to entry of meat and meat products, it has little impact on the overall risk for ASFV (Table 4). Ornithodoros (soft) ticks are the vectors for ASFV with Ornithodoros moubata, O. erraticus and O. sonrai serving as natural vectors in southern Africa, the Iberian Peninsula and recently Senegal, respectively [Reference Vial37]. It should be noted that climate change has brought about the expansion in range of Ornithodoros ticks species. Of particular interest here is that the soft tick, Ornithodoros (formerly Alectorobius) sonrai, has expanded its range south of the Sahara in response to drought, moving with the 750 mm isohyet [Reference Trape38]. Although it could be argued that warmer, drier climates could enable the expansion in range of soft ticks of the genus Ornithodoros within the EU, this would affect the risk of ASFV becoming endemic. Indeed, ASFV-infected ticks may provide a reservoir of the disease, for example through transovarial transmission [Reference Rennie, Wilkinson and Mellor39], making its elimination more problematic, similar to Portugal [40].
The predicted current risks of incursion (Table 4) and becoming endemic (Table 5) are highest for WNV, reflecting the fact that the virus is circulating in some EU countries. WNV is transmitted by mosquito vectors with birds being the host reservoir. Currently, the predicted risks of incursion for WNV through entry of wildlife (Table 6) are greater than that predicted from entry of vectors, livestock, pets and humans. This risk through wildlife may reflect mosquitoes' feeding on infected migratory birds. Indeed, the wide range and large numbers of susceptible species of migratory birds have been important in the dispersal of WNV throughout the Americas [Reference Gould and Higgs4]. Elicitation of expert opinion concluded that the risk of incursion through entry of wildlife is unaffected by climate change (Table 6) suggesting that wild bird movements will be relatively unaffected by climate change. Indeed, climate change may cause a decline in the abundance of some long-distance migratory birds [Reference Both41]. However, of great interest is the prediction that climate change will increase the risk of WNV incursion through entry of vectors (Table 6). It was reported recently that evidence does not exist to support WNV activity in British birds of different species tested, including passerines and corvids [Reference Phipps42], which contradicts previous studies demonstrating WNV serological activity in the UK [Reference Buckley43]. Over the next two decades, the UK may become more permissive to WNV survival and spread [Reference Mellor and Hamblin30]. Factors include increasing mosquito numbers, newly arrived competent mosquito species, newly arrived virulent strains of the disease, and a greater tendency for UK citizens to spend the early evenings outdoors, when they may be bitten in the same sort of situations that appear to be important in the Americas. Simulations of climate change on Usutu virus (which is related to WNV) predict the frequency of outbreaks in blackbirds in the Vienna area of Austria increasing through the 21st century with the virus becoming endemic after 2040 [Reference Brugger and Rubel44]. It should be noted that local anthropogenic changes may also have important impact on the mosquito vectors for WNV in Europe [Reference Poncon45]. In the Camargue region of France, for instance, the abundance of Culex modestus, which is the main mosquito vector for WNV, has been affected by expansion of rice cultivation, pesticide use and pest-management strategies [Reference Poncon45].
The lowest risk range was predicted for RVFV (Tables 4 and 5), which is consistent with RVFV being the only one of the five viruses studied here which has not occurred in the EU to date. Although the median risks are unaffected by climate change, the 10th–90th percentiles for risk of incursion (Table 4) increased from negligible–low to low–medium with climate change, mirroring the increase in risk of incursion of RVFV predicted from entry of vectors (Table 6). The main vectors for RVFV are mosquitoes. During periods of drought, the virus survives in eggs of certain species of Aedes mosquitoes that hatch when rainfall occurs [Reference Reiter9]. It has been suggested that extreme weather events such as floods and droughts, may create the necessary conditions for RVFV to expand its geographical range northwards and across the Mediterranean [Reference Martin46]. RVFV is one of the major vector-borne zoonoses in Africa, and human and animal cases were detected in the Arabian Peninsula for the first time in 2000. A study of the environmental and animal risk factors associated with RVFV in south-west Saudi Arabia identified associations between the disease and a dense mosquito population, high rainfall and the presence of ponds [Reference Elfadil, Hasab-Allah and Dafa-Allah47]. For RVFV, the predicted risk of incursion through entry of livestock is currently greater than for entry of wildlife (Table 6). The completion of a trans-Sahara road will heighten the risk of introduction of RVFV north of the Sahara [Reference Reiter9]. Major trade in animals already exists between Africa and the Arabian Peninsula with an estimated seven million animals exported to Saudi Arabia during the pilgrimage seasons every year. Movement of infected animals and mosquito vectors, in addition to climate change, will determine whether RVFV disperses beyond its current boundaries [Reference Gould and Higgs4].
In an assessment of 45 infectious and parasitic diseases, four vector-borne arboviruses were classified as being of high importance in France [Reference Dufour48]. That study [Reference Dufour48] concluded that with climate change, the probability of the evolution of the epidemiological situation of disease in France was negligible to low for RVFV, low to moderate for AHSV and high for WNV (and BTV). It is difficult to directly compare results, since those of the French study [Reference Dufour48] are relevant to France only and not to the whole EU as is considered here. In addition, the analysis here breaks the risks down into the risk of incursion and risk of becoming endemic.
Breaking down the risks into the specific release routes provides further information to assist risk managers. The risk predictions made here through elicitation of expert opinion should be used in conjunction with consideration of the impact of climate change on vertebrate host reservoirs, arthropod vectors, farming practice and land use, together with an understanding of the biology of virus–vector–host interaction. The two approaches together provide a powerful tool for furthering our understanding of the potential impact of climate change on the emergence of vector-borne viruses, currently exotic to the EU.
ACKNOWLEDGEMENTS
This work was funded under work package 7.4 by EPIZONE, a Network of Excellence for Epizootic Disease Diagnosis and Control. We acknowledge the contributions of Trevor Drew (Veterinary Laboratories Agency, UK) and Ann Lindberg (National Veterinary Institute, Sweden) during the development of the questionnaire. We would like to thank the 18 expert scientists who participated; Paul Phipps (Veterinary Laboratories Agency, UK), Philip Mellor, Linda Dixon, Anthony Wilson (Institute of Animal Health, UK), Norbert Nowotny (University of Veterinary Medicine, Vienna, Austria), Stuart Perkins (Defence Science and Technology Laboratory, UK), Sylvie Lecollinet (Laboratoire d'études et de recherches en pathologie animale et zoonoses, France), Arife Erturk (Etlik Central Veterinary Control and Research Institute, Turkey), Jeroen Kortekaas (Animal Sciences Group, Wageningen University Research, The Netherlands), Paul Reiter (Institut Pasteur, France), Kurt Pfister (Ludwig-Maximilians-Universitaet Muenchen, Germany), Alessandra della Torre (University of Roma La Sapienza, Italy), Gioia Capelli, Paola De Benedictis (Istituto Zooprofilattico Sperimentale delle Venezie, Italy), Ulla Carlsson, Lena Renstrom (National Veterinary Institute, Sweden), Michele Dottori (Istituto Zooprofilattico of Lombardia and the Emilia Romagna, Italy) and Federica Monaco (Istituto Zooprofilattico Sperimentale dell'Abruzzo e del Molise G Caporale, Italy). We thank John Gloster of the Met Office (UK) for his presentation on climate change in Europe at the workshop.
DECLARATION OF INTEREST
None.











