Introduction
Duration of seed dormancy as related to cereal grain end-uses
Cereals are the most important plant source of carbohydrates and proteins for human nutrition. Their many end-uses include bakery, milling, malting and brewery, and direct grain consumption. The cereal grain is also required to perform as the propagule for a new crop. From a botanical point of view, the cereal grain is a caryopsis (i.e. a dry, one-seeded fruit in which the pericarp, derived from the ovary wall, is fused to the seed coat) that can be ‘dressed’ (barley, oat, rice) or ‘naked’ (wheat, sorghum, maize), depending on the presence or not of the lemma, palea and in some cases, even glumes. Modern cereals (wheat, barley, maize, rice, sorghum, rye, triticale) originated from both temperate and tropical areas and developed very diverse responses to environmental factors such as temperature and light quality.
As defined elsewhere, dormant seeds are those that, due to some internal block, cannot germinate in the same conditions (i.e. water, air, temperature) that are suitable for the germination of non-dormant seeds (Bewley and Black, Reference Bewley and Black1994; Bewley, Reference Bewley1997). As a trait, dormancy can be associated with a long list of adaptive advantages: seasonal detection, gap detection, spread of germination in time, depth detection, etc. Therefore, plants living in the wild (including the ancestors of modern cereals) can maximize the survivorship of their descendants due to the fine adjustment between germination opportunity and the environment provided by the different, and often sophisticated, dormancy mechanisms. This evident advantage derived from dormancy clearly disappears in cereal crops since, as with other crop species, emergence timing is carefully controlled to obtain maximum coincidence between plant phenology and environmental conditions that optimize yield and quality. Simultaneous emergence of all individuals is also required to ensure a homogeneous population for proper crop management. Hence, any variation in the timing of emergence because of the possible existence of remnant dormancy in the grain lot that is used to generate a new crop cannot be tolerated under production conditions. In addition, when industrial uses of grain involve germination (i.e. malting) a persistent dormancy precludes grain utilization until dormancy is terminated, thus increasing costs of storage and protection. For all these reasons, high selection pressure must have been exerted against dormancy throughout the domestication process. In some cases, this selection against dormancy has gone too far and grains are germinable even prior to crop harvest. This situation, combined with rainy or damp conditions prevailing during the last stages of maturation, may lead to germination on the mother plant, a phenomenon that is better known as pre-harvest sprouting (PHS). PHS has a wide range of consequences, all of them adverse, which go from the immediate loss of seed viability upon subsequent desiccation when the embryo has grown beyond the point at which it loses its desiccation tolerance, to a large reduction in seed longevity when embryo growth has not proceeded that far (Gualano et al., Reference Gualano, Del Fueyo and Benech-Arnold2014). But, overall, the initiation of germination triggers the synthesis of enzymes that promote reserve mobilization, thus leading to dramatic alterations in grain quality.
The production problems associated with either a short- or a long-lasting dormancy have caught the attention of seed scientists, and thus an important proportion of dormancy research in recent years has been devoted to studies on cereal grains. Clearly, the final aim is to adjust the timing of dormancy loss to a precise time window (i.e. neither as early as to expose the crop to the risk of PHS, nor as late as to have a dormant seed lot at the time of subsequent sowing or industrial utilization). This time window can be particularly narrow in malting barley because the industry demands genotypes with low dormancy at harvest for immediate industrialization. Although some of the dormancy mechanisms operating in the cereal grains closely resemble those described for model organisms (i.e. Arabidopsis and Brachypodium distachyon), the cereals have many particularities and, moreover, different mechanisms appear to prevail in the distinct cereal species. In this sense, any new dormancy mechanism evidenced in a cereal grain must be seen, through its manipulation, as an opportunity to achieve the goal of adjusting the timing of dormancy release to the end-use of that grain. In this paper we review the progress made in our understanding of dormancy in cereals, pointing out similarities and differences between species and, overall, having in mind the possibility of detecting plausible routes to solve the above-mentioned practical problems.
Environmental factors and dormancy expression, induction and release
As in other species, freshly harvested cereal grains are considered to be dormant when they fail to germinate under apparently favourable hydric, gaseous and thermal conditions (Bewley and Black, Reference Bewley and Black1994; Bewley, Reference Bewley1997). Dormancy in cereals is rarely absolute (i.e. no germination at all under any environment), but more often it is a relative phenomenon, and germination of dormant grains can occur only within a narrow range of environmental conditions when compared to non-dormant grains. The way in which the expression of dormancy interacts with environmental factors, such as incubation temperature, depends on the genotype. Dormancy is very common in cereals originating either from temperate (wheat, barley, rye and oat) or from tropical areas (sorghum, rice, millet), while it is almost absent from maize at maturity (reviewed in Simpson, Reference Simpson1990). In temperate cereals, dormancy is expressed strongly (i.e. germination is inhibited) at relatively high temperatures, usually above 15–20°C (Corbineau and Côme, Reference Corbineau and Côme1980, Reference Corbineau and Côme1996; Corbineau et al., Reference Corbineau, Sanchez, Côme and Chaussat1981, Reference Corbineau, Lecat and Côme1986; Benech-Arnold et al., Reference Benech-Arnold, Gualano, Leymarie, Côme and Corbineau2006). On the contrary, dormant seeds from tropical cereals reach higher germination values at high incubation temperatures, the optimum being close to 25–30°C, while they germinate poorly (i.e. dormancy is expressed) at lower temperatures (Simpson, Reference Simpson1990; Benech-Arnold et al., Reference Benech-Arnold, Enciso, Sanchez and Rodriguez2003). Dormant grains can also be very sensitive to other factors that inhibit germination, such as oxygen deprivation (Corbineau and Côme, Reference Corbineau and Côme1980; Corbineau et al., Reference Corbineau, Sanchez, Côme and Chaussat1981, Reference Corbineau, Lecat and Côme1986; Lenoir et al., Reference Lenoir, Corbineau and Côme1983, Reference Lenoir, Corbineau and Côme1986; Bradford et al., Reference Bradford, Côme and Corbineau2007, Reference Bradford, Benech-Arnold, Côme and Corbineau2008), low water potential of the medium (Corbineau and Côme, Reference Corbineau and Côme1996) and exposure to light, in particular blue light (Chaussat and Zoppolo, Reference Chaussat and Zoppolo1983; Gubler et al., Reference Gubler, Hughes, Waterhouse and Jacobsen2008; Jacobsen et al., Reference Jacobsen, Barrero, Hughes, Julkowska, Taylor, Xu and Gubler2013; Barrero et al., Reference Barrero, Downie, Xu and Gubler2014; Hoang et al., Reference Hoang, Sechet, Bailly, Leymarie and Corbineau2014).
Although dormancy is a heritable trait, its intensity at harvest and its maintenance afterwards can also be modulated by the environmental conditions throughout grain development and maturation, and during grain storage. Among the different factors acting on the mother plant, temperature appears as a major factor responsible for year-to-year variation in grain dormancy (and sprouting susceptibility) for plants with the same genotype. Low temperatures during grain development result in higher grain dormancy in wheat (Black et al., Reference Black, Butler, Hughes and Mares1987; Nakamura et al., Reference Nakamura, Abe, Kawahigashi, Nakazono, Tagiri, Matsumoto, Utsugi, Ogawa, Handa, Ishida, Mori, Kawaura, Ogihara and Miura2011) and barley (Rodríguez et al., Reference Rodriguez, González Martín, Insausti, Margineda and Benech-Arnold2001). The quantitative relationship between temperature during grain development and the level of dormancy soon after physiological maturity was explored in malting barley, showing that a positive linear relationship exists between the average temperature at the crop site during a precise ‘thermal time window’ and the germination index of grains tested 12 d after physiological maturity (Rodríguez et al., Reference Rodriguez, González Martín, Insausti, Margineda and Benech-Arnold2001). This predictive model was later adapted to several malting barley cultivars in Argentina and, together with local weather forecast data, provides a tool for estimating the sprouting risk for a particular crop and for making crop-management decisions (Rodríguez et al., Reference Rodriguez, González Martín, Insausti, Margineda and Benech-Arnold2001; Gualano and Benech-Arnold, Reference Gualano and Benech-Arnold2009a). The impact of nitrogen and water availability has also been evaluated (Gualano and Benech-Arnold, Reference Gualano and Benech-Arnold2009b). In this study, drought promoted dormancy release but the magnitude of this effect depended on the thermal conditions during the sensitivity window described above. A different result was obtained for a low-dormancy wheat (Biddulph et al., 2005), in which drought treatments during grain maturation increased dormancy and sprouting tolerance, overriding the effects of temperature. Understanding these genetics × environment (G × E) interactions for different crops is important to aid breeding for adequate dormancy responses under particular environments.
After harvest, dormancy is gradually lost during dry storage at ambient temperatures. This phenomenon, usually termed ‘afterripening’, results in an increased germination rate at the thermal optimum and widening of the temperature range compatible with good germination (Corbineau and Côme, Reference Corbineau and Côme1980, Reference Corbineau and Côme1996; Corbineau et al., Reference Corbineau, Sanchez, Côme and Chaussat1981; Côme et al., Reference Côme, Lenoir and Corbineau1984). During dormancy release, grains also become less sensitive to the inhibitory effects of hypoxia (Corbineau and Côme, Reference Corbineau and Côme1980; Bradford et al., Reference Bradford, Côme and Corbineau2007, Reference Bradford, Benech-Arnold, Côme and Corbineau2008) and light (Chaussat and Zoppolo, Reference Chaussat and Zoppolo1983; Gubler et al., Reference Gubler, Hughes, Waterhouse and Jacobsen2008; Barrero et al., Reference Barrero, Talbot, White, Jacobsen and Gubler2009). Using a population-based threshold model, Bradford et al. (Reference Bradford, Côme and Corbineau2007, Reference Bradford, Benech-Arnold, Côme and Corbineau2008) have quantified the median base O2 percentage that allows 50% germination; it was estimated to be 2.27% and 0.05%, respectively, for wheat grains before and after ripening, and 36.30% and 0.30%, respectively, for barley grains at harvest and after 9.5 months of storage at 20°C.
Afterripening usually occurs at ambient temperatures, but loss of dormancy can be sped up by increasing the temperature during storage (Simpson, Reference Simpson1990). Conversely, afterripening is slowed down and dormancy is maintained when seeds are stored dry in a freezer at − 18°C (Lenoir et al., Reference Lenoir, Corbineau and Côme1983) and this has been a useful resource in dormancy research, providing ready accesss to dormant grains.
Induction of secondary dormancy
Primary dormancy is set during seed development, but a secondary dormancy can develop in response to conditions unfavourable for germination in mature cereal grains which retain some degree of primary dormancy (Hilhorst, Reference Hilhorst, Bradford and Nonogaki2007; Hilhorst et al., Reference Hilhorst, Finch-Savage, Buitink, Bolingue, Leubner-Metzger, Lubzens, Cerdà and Clarck2010). Induction of secondary dormancy by high temperature and hypoxia (e.g. produced by immersion of seeds in water) occurs in numerous cereals (Simpson, Reference Simpson1990), such as wheat (Grahl, Reference Grahl1965), barley (Leymarie et al., Reference Leymarie, Robayo-Romero, Gendreau, Benech-Arnold and Corbineau2008; Hoang et al., Reference Hoang, Bailly, Corbineau and Leymarie2013a, Reference Hoang, Sotta, Gendreau, Bailly, Leymarie and Corbineaub) and oat (Corbineau et al., Reference Corbineau, Black and Côme1993; Corbineau and Côme, Reference Corbineau, Côme, Nicolas, Bradford, Côme and Pritchard2003). In barley and oat, induction of a thermodormancy is already apparent after 3–8 h of incubation at 30°C, and is optimal after 1–3 d (Corbineau et al., Reference Corbineau, Black and Côme1993; Leymarie et al., Reference Leymarie, Robayo-Romero, Gendreau, Benech-Arnold and Corbineau2008). This phenomenon corresponds to a reinforcement of the primary dormancy, grains being able to germinate in a narrower temperature range than those having primary dormancy. The induction of thermodormancy requires a critical moisture content of the embryo, of about 40–50% (dry weight basis) (Corbineau and Côme, Reference Corbineau, Côme, Nicolas, Bradford, Côme and Pritchard2003; Hoang et al., Reference Hoang, Sotta, Gendreau, Bailly, Leymarie and Corbineau2013b), corresponding to an energy charge [EC = (ATP +0.5 ADP)/(ATP + ADP + AMP)] of at least 0.6 (Corbineau and Côme, Reference Corbineau, Côme, Nicolas, Bradford, Côme and Pritchard2003), and concomitant with activation of the cell cycle (Gendreau et al., Reference Gendreau, Romaniello, Barad, Leymarie, Benech-Arnold and Corbineau2008; Hoang et al., Reference Hoang, Sotta, Gendreau, Bailly, Leymarie and Corbineau2013b). More recently, Hoang et al. (Reference Hoang, Bailly, Corbineau and Leymarie2013a, Reference Hoang, Sechet, Bailly, Leymarie and Corbineau2014) have also demonstrated that secondary dormancy can be imposed during imbibition at lower temperatures (10–15°C) combined with oxygen tensions lower than 10% or under blue light, conditions that inhibit germination of seeds having primary dormancy.
Anatomy of cereal seed dormancy
Grain structure and chemical composition of the coats
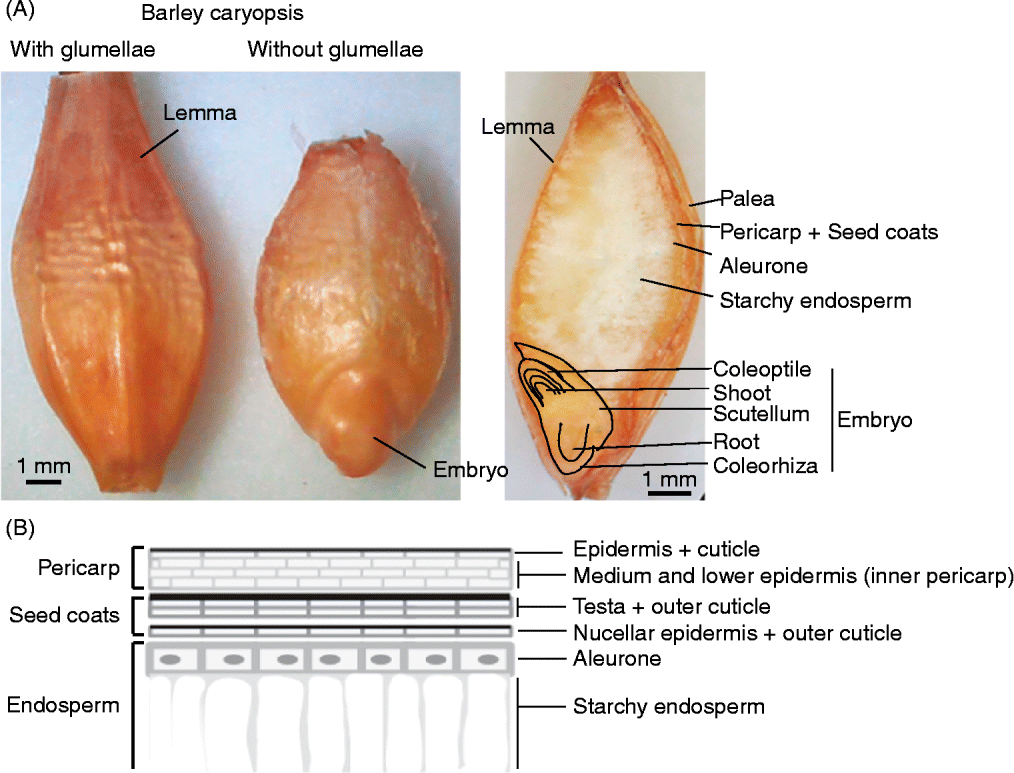
The dispersal unit of cereals is a caryopsis, i.e. a dry monospermic indehiscent fruit, where the pericarp is fused to the outside of the true seed coat (testa) (Fig. 1). The grain comprises (1) an embryo, consisting of the embryonic axis with the coleorhiza surrounding the radicle (or embryonic roots) and the coleoptile enclosing the shoot meristem and primary leaves, and the scutellum; (2) the endosperm, differentiated into two layers consisting of the starchy endosperm and the surrounding aleurone layer, constituted by one (wheat, rye, maize, oat, sorghum) or up to three (barley) layers of thick-walled cells; (3) the seed coats (nucellus and testa) fused to the pericarp (Simpson, Reference Simpson1990; Kent and Evers, Reference Kent and Evers1994; Evers and Nesbitt, Reference Evers, Nesbitt, Black, Bewley and Halmer2006). Endosperm and embryo are surrounded by the nucellar epidermis with a cuticle on the outer side in most cereals, and the testa with one or two cellular layers, except in some sorghum varieties where the testa is absent. An external cuticle can also be present on the outer surface of the testa in some cereals such as rice, oat and maize, regulating water absorption. The pericarp is a multilayered structure with empty dead cells, which can differentiate epidermal trichomes at the non-embryo end in wheat (brush), barley and oat. In addition, in barley, oat, rice and some sorghum genotypes, the grain is covered to different extents by the hulls (consisting of the glumellae – lemma and palea – or the glumes, depending on the species; Fig. 2) which are not removed (barley, oat), are removed with difficulty (rice), or are more or less easily (sorghum) removed by threshing. These bracts, also referred to as hulls, constitute an additional covering structure that affects water uptake and oxygen supply to the embryo, and may also supply water-soluble chemicals to the caryopsis. The presence of these bracts may vary between the intact spikelet (before harvest) and after harvest and threshing (Fig. 2); therefore, the contribution of these bracts, either to PHS tolerance or to germination of the threshed grains, has to be considered accordingly.
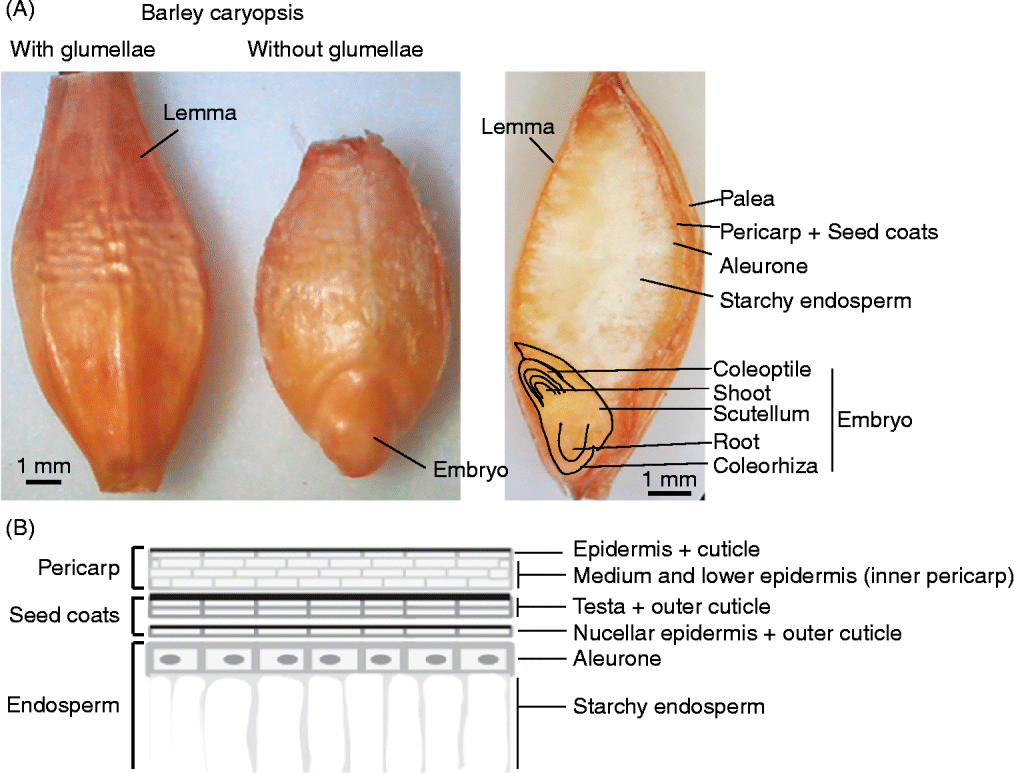
Figure 1 (A) Dorsal view of a barley grain with the hulls (glumellae) and after manual removal of the lemma and palea. On the right, a longitudinal cross-section of a barley grain indicating the main structures. (B) Schematic view of the external layers of a caryopsis, including the pericarp, the seed coats (testa plus nucellar epidermis, each with an outer cuticle) and the aleurone layer (living endosperm) above the starchy endosperm (dead cells, with amyloplasts). The number of cells for each of these layers varies among different species (see text), as does the composition and distribution of a variety of phenolic compounds with antioxidant properties, which can confer pigmentation.
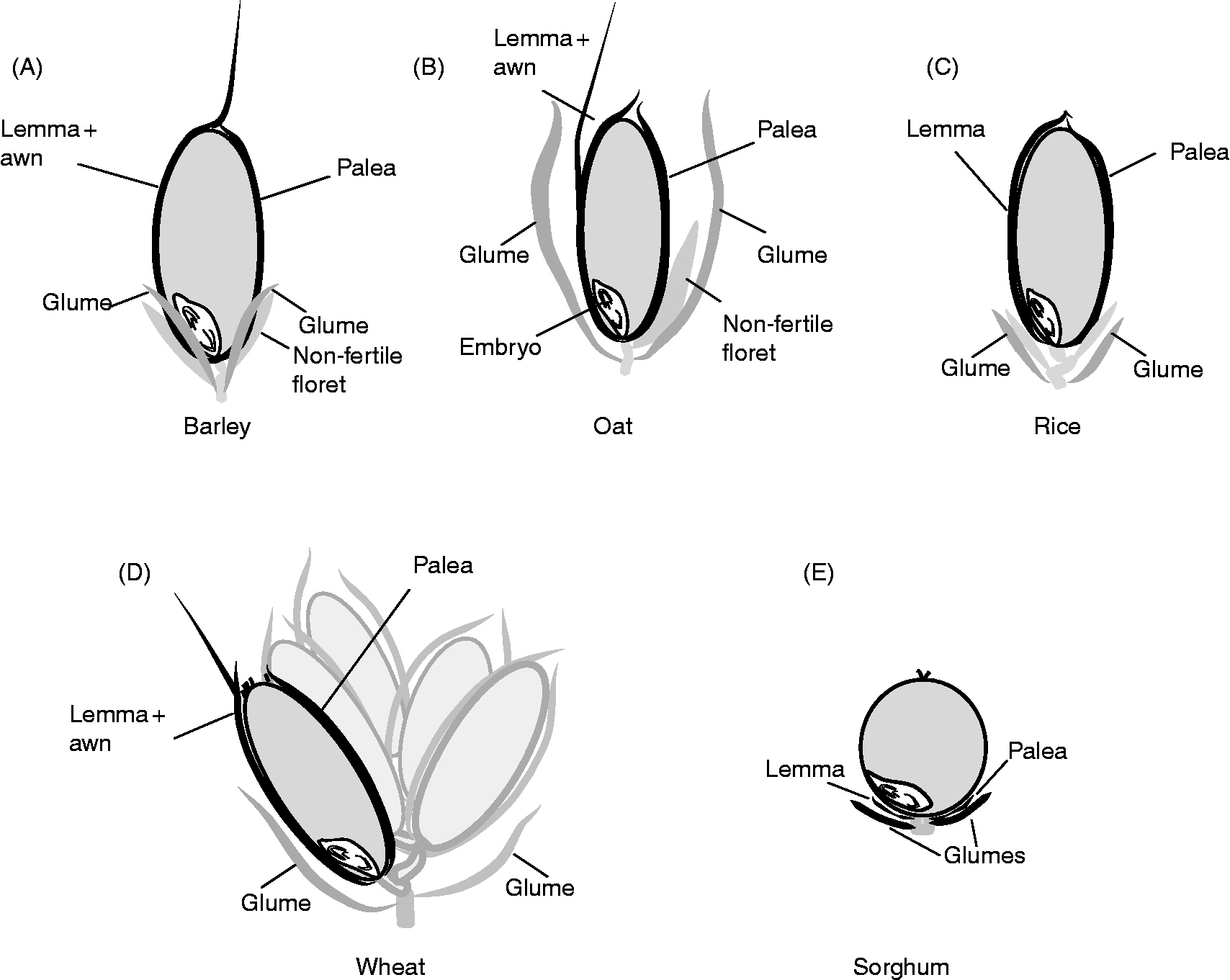
Figure 2 Schematic view of spikelets from different cereal species, indicating (in black) the hulls (or bracts) that may remain attached to the mature caryopsis. In both barley (A; two-row barley, one fertile floret develops into the grain) and oat (B) the caryposis is enclosed by the lemma and palea that remain in the detached grain, and these bracts are not removed by threshing. In both rice (C) and wheat (D; multiple grains in one spikelet) the lemma and palea, which are loosely attached to the mature caryopses, are usually removed mechanically during threshing. In the sorghum grain (E) both glumellae and glumes may vary greatly in size, depending on the genotype. When these bracts are short, removal is easy during harvest and threshing, but this becomes more difficult with increasing size of the glumes (which in some cases cover the entire caryopsis).
The external structures of cereal grains, including testa, pericarp and hulls, accumulate various phenolic compounds, such as phenolic acids (sinapic-, ferulic-, caffeic-, vanillic- and p-hydroxybenzoic acid), coumarins, flavonoids and tannins (Jayachandran-Nair and Sridhar, Reference Jayachandran-Nair and Sridhar1975; Slominski, Reference Slominski1980; Glennie, Reference Glennie1981; Collins, Reference Collins and Webster1986; Lenoir et al., Reference Lenoir, Corbineau and Côme1986; Weidner et al., Reference Weidner, Paprocka, Kamieniecki, Zadernowski, Walker-Simmons and Ried1993, Reference Weidner, Amarowicz, Karamac and Dabrowski1999, Reference Weidner, Amarowicz, Karamac and Fraczek2000, Reference Weidner, Krupa, Amarowicz, Karamac and Abe2002; Tian et al., Reference Tian, Nakamura and Kayara2004). The major flavonoids are flavonols, anthocyanins, deoxyflavonoids (or phlobaphenes) and proanthocyanidins (Winkel-Shirley, Reference Winkel-Shirley1998, Reference Winkel-Shirley2001; Lepiniec et al., Reference Lepiniec, Debeaujon, Routaboul, Baudry, Pourcel, Nesi and Caboche2006). Flavan-4-ols are the major precursors of phlobaphenes which confer a red colour in wheat, maize and rice (Grotewold et al., Reference Grotewold, Drummond, Bowen and Peterson1994; Himi et al., Reference Himi, Mares, Yanagisawa and Noda2002), and condensation of flavan-3-ols results in synthesis of proanthocyanidins, the oxidation of which (through polyphenol oxidase activity) confers a brown colour (Winkel-Shirley, Reference Winkel-Shirley1998). In rice, flavonoids and proanthocyanidins confer red colour to the grains (Oki et al., Reference Oki, Masuda, Kobayashi, Nishiba, Furuta, Suda and Sato2002; Sweeney et al., Reference Sweeney, Thomson, Pfeil and McCouch2006), and accumulate only in the lower epidermal cell layer of the pericarp (Gu et al., Reference Gu, Foley, Horvath, Anderson, Feng, Zhang, Mowry, Ye, Suttle, Kadowaki and Chen2011). Purple and black pigments in rice grains are also associated with the presence of anthocyanins (Reddy et al., Reference Reddy, Dash and Reddy1995).
Several of the above-mentioned phenolics were tested in germination assays and found to have a strong inhibitory effect on cereal grains, including wheat, barley and sorghum (Krogmeier and Bremner, Reference Krogmeier and Bremner1989). It has been proposed that some of these soluble components diffuse from the coats into the imbibing embryo and contribute to coat-based dormancy in wheat (discussed in Rathjen et al., Reference Rathjen, Strounina and Mares2009). Although phenolic compounds in the grain may perform as germination inhibitors themselves, studies that quantify their effects in vivo and in relation to dormancy are still lacking. On the other hand, it is known that these components in the hulls have a role in regulating oxygen levels in barley and oat caryopses, as will be discussed in the following section.
Dormancy imposed by the seed-covering structures
Studies performed with the main genera of cereals demonstrate that the inability of the grains to germinate at harvest is due to a complex interplay between coat- and embryo-based dormancy, with the former playing a major role in the overall contribution to grain dormancy. Removal of the covering structures, such as the hulls (glumellae or glumes) or the pericarp, testa and endosperm from the caryopsis results in a reduced expression of dormancy at a wide range of temperatures (Côme et al., Reference Côme, Lenoir and Corbineau1984; Simpson, Reference Simpson1990; Corbineau and Côme, Reference Corbineau, Côme, Côme and Corbineau1993, Reference Corbineau and Côme1996; Steinbach et al., Reference Steinbach, Benech-Arnold, Kristof, Sanchez and Marcucci-Poltri1995; Benech-Arnold et al., Reference Benech-Arnold, Enciso, Sanchez and Rodriguez2003; Benech-Arnold, Reference Benech-Arnold, Benech-Arnold and Sanchez2004). Nevertheless, although the naked caryopsis or the isolated embryos might appear to be non-dormant when incubated in water, different responsiveness to inhibitory factors such as abscisic acid (ABA) or hypoxia (which are speculated to be maintained by the covering structures) suggest that embryo-related factors (discussed in the section ‘Hormones in the expression of dormancy in the imbibed grain’ below) are also relevant in the expression of coat-imposed dormancy. An example of this contribution of the embryo to grain dormancy was provided by genetic studies in wheat by Flintham (Reference Flintham2000), who identified an embryo-related factor that increases dormancy and tolerance to PHS in both red and white varieties.
The intensity of coat-based inhibition of germination depends on the species or cultivar, the environmental conditions of seed development on the ear, and the maturity stages of the grains. In barley, the glumellae have a main role in dormancy, since de-hulled grains germinate in a wide range of temperatures (Lenoir et al., Reference Lenoir, Corbineau and Côme1983, Reference Lenoir, Corbineau and Côme1986; Corbineau and Côme, Reference Corbineau and Côme1996), while in oat, the glumellae together with the testa plus pericarp participate in dormancy (Corbineau et al., Reference Corbineau, Lecat and Côme1986). In barley, the presence of hulls is a desirable trait as they aid during the filtration phase in the brewing process. In naked grains, such as wheat and sorghum, the pericarp, testa and endosperm surrounding the embryo also contribute to dormancy (Corbineau et al., Reference Corbineau, Sanchez, Côme and Chaussat1981; Steinbach et al., Reference Steinbach, Benech-Arnold, Kristof, Sanchez and Marcucci-Poltri1995), and dormancy has often been associated with grain colour. In temperate cereals (e.g. barley and oat), the inhibitory effect of the covering structures is very weak at low incubation temperatures (5–10°C), while it increases at higher temperatures (Lenoir et al., Reference Lenoir, Corbineau and Côme1983, Reference Lenoir, Corbineau and Côme1986; Corbineau et al., Reference Corbineau, Sanchez, Côme and Chaussat1981, Reference Corbineau, Lecat and Côme1986; Corbineau and Côme, Reference Corbineau and Côme1996). This coat-imposed dormancy gradually disappears during afterripening (Côme et al., Reference Côme, Lenoir and Corbineau1984).
The covering structures mainly inhibit germination by limiting oxygen supply to the embryo; although isolated embryos of dormant grains germinate fully when incubated in a 3–5% oxygen atmosphere, the oxygen levels beneath the glumellae can drop below these values depending on incubation temperature and glumella properties. Oxygen levels within the imbibed grain decrease as a result of respiratory activity in the embryo and aleurone layer, but also due to oxidative reactions in the hulls (Lenoir et al., Reference Lenoir, Corbineau and Côme1986; Corbineau and Côme, Reference Corbineau and Côme1996; Benech-Arnold et al., Reference Benech-Arnold, Gualano, Leymarie, Côme and Corbineau2006; Bradford et al., Reference Bradford, Benech-Arnold, Côme and Corbineau2008; Hoang et al., Reference Hoang, Bailly, Corbineau and Leymarie2013a). Recently, Hoang et al. Reference Hoang, Bailly, Corbineau and Leymarie(2013a) demonstrated that the embryo oxygen content under the glumellae, measured with micro-sensors, is very much lower at 30°C (0.3%), a temperature at which barley grain dormancy is expressed, than at 15°C (15.8%), at which grains germinate. This limitation to oxygen penetration by the covering structures is also supported by a 400-fold greater median oxygen requirement for germination of dormant intact grains than for isolated embryos (Bradford et al., Reference Bradford, Benech-Arnold, Côme and Corbineau2008).
During imbibition, the hulls act as a barrier to oxygen diffusion into the embryo since oxygen has to dissolve in the imbibed water, but glumellae also provide substrates and enzymes for oxidation reactions. In barley and oat, the glumellae fix abundant amounts of oxygen during imbibition through enzymatic oxidation of phenolic compounds by polyphenol oxidases localized in the cell walls (Corbineau et al., Reference Corbineau, Lecat and Côme1986; Lenoir et al., Reference Lenoir, Corbineau and Côme1986). The efficiency of this oxygen-depleting mechanism rises with increasing temperature; at 30°C, for example, more than 50% of the total oxygen absorbed by the grain is accounted for by the glumellae, while it does not exceed 25% at 10–15°C (Lenoir et al., Reference Lenoir, Corbineau and Côme1983; Côme et al., Reference Côme, Corbineau and Lecat1988). There are, however, practically no qualitative or quantitative differences in phenol and polyphenol oxidase compositions between glumellae of dormant and non-dormant caryopses. Instead, the temporal pattern of oxygen uptake (but not the total amount of absorbed oxygen) has been related to the different germination response of fresh (dormant) and afterripened barley grains. Glumellae of dormant grains start to take up oxygen at the beginning of imbibition, whereas those of non-dormant grains start to take up oxygen only after about 12 h and maintain higher oxygen levels within the grain during early imbibition (Lenoir et al., Reference Lenoir, Corbineau and Côme1983, Reference Lenoir, Corbineau and Côme1986; Corbineau et al., Reference Corbineau, Lecat and Côme1986). It has been suggested that progressive and spontaneous cross-linking of substrates and/or enzymes to the cell wall during afterripening might delay their solubility upon hydration, providing a causal relationship between afterripening, changes in the timing of oxygen uptake and dormancy loss (Lenoir et al., Reference Lenoir, Corbineau and Côme1983).
Phenolic compounds in the testa and/or pericarp of grains may also influence the regulation of oxygen levels in the embryo. Red-grained wheat and rice cultivars usually show a higher level of dormancy than white-grained ones (Gordon, Reference Gordon1979; Cohn and Hughes, Reference Cohn and Hughes1981; Mares, Reference Mares1993; Flintham, Reference Flintham2000), and pigmentation of the testa and dormancy are also positively related in barley grains (Himi et al., Reference Himi, Yamashita, Haruyama, Yanagisawa, Maekawa and Taketa2012). Genetic analysis has also associated colour of the pericarp with seed dormancy in weedy red rice (Gu et al., Reference Gu, Kianian and Foley2005, Reference Gu, Foley, Horvath, Anderson, Feng, Zhang, Mowry, Ye, Suttle, Kadowaki and Chen2011), wheat (Himi et al., Reference Himi, Mares, Yanagisawa and Noda2002) and in the model plant species Arabidopsis thaliana, where the transparent testa mutants further support a link between testa pigmentation and dormancy (Debeaujon et al., Reference Debeaujon, Leon-Kloosterziel and Koornneef2000, Reference Debeaujon, Peeters, Leon-Kloosterziel and Koornneef2001). Normal pigmentation of the testa is also necessary for normal expression of dormancy in barley: the mutant ant28 fails to accumulate proanthocyanidins in the testa and displays reduced grain dormancy (Himi et al., Reference Himi, Yamashita, Haruyama, Yanagisawa, Maekawa and Taketa2012). Through molecular cloning of barley ant28, Himi et al. (Reference Himi, Yamashita, Haruyama, Yanagisawa, Maekawa and Taketa2012) showed that it encodes Tamyb10, a transcriptional regulator previously identified in wheat as the red pericarp (R-1) gene (Himi et al., Reference Himi, Mares, Yanagisawa and Noda2002). Nevertheless, the physiological mechanism underlying the simultaneous regulation of pigmentation and dormancy by Tamyb10/Hvant28 is still unclear, and it might involve the accumulated pigments directly (which may either inhibit germination themselves or through the regulation of oxygen levels) or the regulation of another ‘dormancy’ gene by this transcription factor (Himi et al., Reference Himi, Yamashita, Haruyama, Yanagisawa, Maekawa and Taketa2012).
In addition to their role in the seed coats and hulls, phenolic components accumulated within the embryo might also have a role in the expression of embryo-related dormancy; an inverse correlation between the contents of free phenolic acids in developing embryos and the intensity of precocious germination occurs in triticale (Weidner et al., Reference Weidner, Krupa, Amarowicz, Karamac and Abe2002).
Hormonal regulation of dormancy
The ABA–GA antagonism regulates dormancy in the developing cereal grain
The plant hormone abscisic acid (ABA) has a crucial role in the induction of dormancy in the developing grains of all cereal species studied so far. It plays a central role in higher plants by regulating plant growth and development, including seed maturation and dormancy, as well as adaptation to a variety of environmental stresses (Zeevaart and Creelman, Reference Zeevaart and Creelman1988; Cutler et al., Reference Cutler, Rodriguez, Finkelstein and Abrams2010; Nambara et al., Reference Nambara, Okamoto, Tatematsu, Yano, Seo and Kamiya2010). Biochemical studies and analyses of mutants that block ABA synthesis indicate that ABA is synthesized by oxidative cleavage of C40 epoxy-carotenoids to produce xanthoxin, which is subsequently converted to ABA via the ABA-aldehyde intermediate (Tan et al., Reference Tan, Schwartz, Zeevaart and McCarty1997; also reviewed in Nambara and Marion-Poll, Reference Nambara and Marion-Poll2003). The cleavage of 9-cis-epoxycarotenoids to xanthoxin, is catalysed by 9-cis-epoxycarotenoid dioxygenases (NCED), and is considered to be the key regulatory step of abscisic acid (ABA) biosynthesis in plants. Catabolism of ABA is also important in regulating ABA levels and comprises two processes: ABA hydroxylation or conjugation. However, the hydroxylation of ABA at the 8′-position to produce 8′-hydroxy ABA, which isomerizes spontaneously to phaseic acid (PA), is considered to be the predominant pathway of ABA catabolism (Nambara and Marion-Poll, Reference Nambara and Marion-Poll2003). ABA inactivation by hydroxylation is performed by members of the CYP707A family of P450 mono-oxygenases, ABA 8′-hydroxylases. All plant species examined to date encode NCED and CYP707A as multigene families, and differential combinations of these members contribute to tissue- and environment-specific regulation (reviewed in Nambara et al., Reference Nambara, Okamoto, Tatematsu, Yano, Seo and Kamiya2010).
Several studies have focused on the evolution of embryonic ABA content during grain development in wheat, sorghum and barley (Walker-Simmons, Reference Walker-Simmons1987; Steinbach et al., Reference Steinbach, Benech-Arnold and Sánchez1997; Benech-Arnold et al., Reference Benech-Arnold, Giallorenzi, Frank and Rodríguez1999), expecting to find less ABA in genotypes displaying an anticipated release from dormancy. However, ABA peaked at around physiological maturity and declined thereafter, and the ABA content of developing grains did not correlate with a particular time of exit from dormancy. In parallel, manipulation of ABA content produced deep changes in patterns of dormancy release. Reduction of ABA during seed development (as in mutants impaired in ABA biosynthesis, or after the application of an ABA-synthesis inhibitor) led to complete abolishment of dormancy or to a reduction of dormancy intensity. For example, maize kernels are viviparous if the ABA signal is reduced or abolished (as in vp5 and vp1 mutants, which are ABA deficient and ABA insensitive, respectively, or with the application of fluridone, an ABA biosynthesis inhibitor) (White and Rivin, Reference White and Rivin2000; White et al., Reference White, Proebsting, Hedden and Rivin2000). Spraying young sorghum panicles with fluridone reduced ABA content in the grains and anticipated dormancy release, making a sprouting-resistant line behave as a sprouting-susceptible one (Steinbach et al., Reference Steinbach, Benech-Arnold and Sánchez1997). Several rice mutants exhibit vivipary and photo-oxidation in the light. These mutants, named phs1 to phs4, result from mutations in genes encoding phytoene desaturase, ζ-carotene desaturase, carotenoid isomerase and lycopene β-cyclase, which are the major enzymes involved in the synthesis of the carotenoid precursors of ABA (Fang et al., Reference Fang, Chai, Qian, Li, Tang, Sun, Huang, Guo, Sun, Liu, Zhang, Lu, Wang, Lu, Han, Chen, Cheng and Chu2008). Evidence for a central role of ABA in the expression of grain dormancy has also been provided by several non-dormant wheat mutants, which display either reduced ABA sensitivity or reduced ABA content (Kawakami et al., Reference Kawakami, Miyake and Noda1997; Rikiishi & Maekawa, Reference Rikiishi and Maekawa2010; Schramm et al., Reference Schramm, Nelson and Steber2012).
Although it became clear from these and many other experiments that manipulating ABA has an impact on dormancy, differences in endogenous ABA content in cereal varieties do not usually appear to be linked to the observed differences in dormancy (Walker-Simmons, 1987; Steinbach et al., Reference Steinbach, Benech-Arnold and Sánchez1997). Instead, responsiveness of isolated embryos to exogenous ABA is highly correlated with dormancy expressed in the intact grains from several species, such as wheat (Walker-Simmons, Reference Walker-Simmons1987; Morris et al., Reference Morris, Moffatt, Sears and Paulsen1989; Corbineau et al., Reference Corbineau, Benamar and Côme2000; Gerjets et al., Reference Gerjets, Scholefield, Foulkes, Lenton and Holdsworth2010), barley (Wang et al., Reference Wang, Heimovaara-Dijkstra and Van Duijn1995; Benech-Arnold et al., Reference Benech-Arnold, Giallorenzi, Frank and Rodríguez1999, Reference Benech-Arnold, Gualano, Leymarie, Côme and Corbineau2006; Leymarie et al., Reference Leymarie, Robayo-Romero, Gendreau, Benech-Arnold and Corbineau2008; Barrero et al., Reference Barrero, Talbot, White, Jacobsen and Gubler2009), oat (Corbineau et al., Reference Corbineau, Poljakoff-Mayber and Côme1991, Reference Corbineau, Black and Côme1993), sorghum (Steinbach et al., Reference Steinbach, Benech-Arnold, Kristof, Sanchez and Marcucci-Poltri1995) and rice (Gianinetti and Vernieri, Reference Gianinetti and Vernieri2007). Different embryo sensitivity to ABA can result from differential signalling activity or efficiency of molecular components of the ABA signal transduction pathway, although differential catabolism rates might also affect embryo responsiveness to exogenous ABA (Benech-Arnold et al., Reference Benech-Arnold, Gualano, Leymarie, Côme and Corbineau2006). The first ABA signalling component identified in cereals was VIVIPAROUS-1 (VP1). Maize vp1 mutant kernels are viviparous (i.e. growth arrest is never imposed in this mutant and the embryo continues to grow), and although ABA amounts are similar to those of the wild type, mutant embryos have reduced sensitivity to ABA (Robichaud and Sussex, Reference Robichaud and Sussex1986). The VP1 gene is expressed in developing seeds, and encodes a transcriptional factor that is a key determinant of seed-specific gene expression, and regulates positively ABA signalling (McCarty et al., Reference McCarty, Carson, Stinard and Robertson1989). The orthologous genes for maize VP1 were also cloned in several cultivars from wheat, rice and sorghum, and several studies have attempted to relate their expression to dormancy. In two grain sorghum inbred lines with contrasting dormancy, expression of VP1 in embryos of developing grains did not differ substantially (Carrari et al., Reference Carrari, Perez-Flores, Lijavetzky, Enciso, Sánchez, Benech-Arnold and Iusem2001). However, in wheat and rice, evidence of missplicing was obtained for genotypes displaying early dormancy release (Fan et al., Reference Fan, Niu, Wang, Ren, Zhuo, Yang, Lu and Liu2007).
Another protein, Sdr4, is involved in the natural variability of dormancy in rice. This protein plays a key role in the establishment of dormancy, and different alleles (Sdr4-k and Sdr4-n) exist in Kasalath (prolonged dormancy) and Nipponbare (brief dormancy) cultivars (Sugimoto et al., Reference Sugimoto, Takeuchi, Ebana, Miyao, Hirochika, Hara, Ishiyama, Kobayashi, Ban, Hattori and Yano2010). Although the precise function of the Sdr4 protein remains unknown, it is clearly related to ABA signalling during seed development: not only is the expression of the Sdr4 gene up-regulated by OsVP1 transcription factor in the developing embryo, but Sdr4 protein is also necessary for ABA-mediated inhibition of germination, and sensitivity to exogenous ABA is enhanced in grains expressing the strong dormancy allele, Sdr4-k.
As mentioned above, red grain colour in wheat and rice, and accumulation of tannins in the testa in sorghum, are positively related to grain dormancy, at least until late maturation stages. Although ABA and flavonoid biosynthetic pathways do not share any common precursor (as do ABA and carotenoids), there are some regulatory components that connect ABA metabolism and/or signalling to flavonoid metabolism. As will be referred to in later sections, this functional connection between grain colour and ABA metabolism in the developing grain has been a problem for breeders seeking to improve dormancy in white-grained cereals without altering their colour. The reason for the tight linkage between these two traits in red rice has been elucidated: a gene responsible for strong dormancy was identified in weedy red rice and found to encode a transcription factor, SD7-1, that regulates several ABA synthesis genes. Normal expression of the SD7-1 allele in red rice results in a peak of ABA around 10 d after pollination, at the same time as it enhances the expression of genes involved in flavonoid biosynthesis and pigmentation of the pericarp (Gu et al., Reference Gu, Foley, Horvath, Anderson, Feng, Zhang, Mowry, Ye, Suttle, Kadowaki and Chen2011). This finding shows that, in red rice, a single gene encoding a transcriptional regulator has pleiotropic effects on dormancy and pigmentation. Similarly, the red pericarp R-gene responsible for grain colour in red wheat (also related to PHS tolerance) encodes a Myb transcription factor (Tamyb10) that promotes dormancy and also the expression of several genes involved in the flavonoid biosynthesis pathway in developing wheat grains (Himi et al., Reference Himi, Mares, Yanagisawa and Noda2002, Reference Himi, Nisar and Noda2005). Nevertheless, in contrast to SD7-1, no evidence regarding an effect of Tamyb10 on ABA metabolism or embryo responsiveness to this hormone has been shown in wheat (Himi et al., Reference Himi, Maekawa, Miura and Noda2011) or barley (Himi et al., Reference Himi, Yamashita, Haruyama, Yanagisawa, Maekawa and Taketa2012) and the mechanism by which it exerts its effect on dormancy is still unknown.
It has been known for a long time that gibberellins (GA) promote germination of dormant seeds in many species, antagonizing the inhibitory effect of ABA. Both ABA and GA also act antagonistically in the inception of dormancy during early development. Evidence for a role of GA in early development was produced by White and co-workers (White and Rivin, Reference White and Rivin2000; White et al., Reference White, Proebsting, Hedden and Rivin2000), who studied developing caryopses of maize mutants impaired in ABA and GA metabolism. Vivipary observed in maize kernels with reduced ABA content (as in the vp-5 mutant, or after treatment with ABA biosynthesis inhibitor) was prevented and the ‘normal’ dormancy phenotype restored in the vp5/d1 double mutant, impaired in both ABA and GA synthesis. These kernels also survived desiccation and were able to germinate at maturity. It can be concluded that the balance between ABA and GA action governs dormancy induction and germination response, and that if ABA signalling is reduced, then germination no longer depends on GA. Similarly, application of a GA biosynthesis inhibitor to young sorghum panicles reduced GA content during development and delayed dormancy release for several weeks in a typically short-lived dormancy line (Steinbach et al., Reference Steinbach, Benech-Arnold and Sánchez1997). Similar to observations on ABA in developing grains of sorghum varieties with contrasting dormancy, no correlation has been found between natural endogenous GA content and dormancy (Benech-Arnold et al., Reference Benech-Arnold, Enciso, Sánchez, Carrari, Pérez-Flores, Iusem, Steinbach, Lijavetzky, Bottini, Black, Bradford and Vázquez Ramos2000).
Gibberellin signalling involves several components, including the DELLA (Asp–Glu–Leu–Leu–Ala) domain repressors of GA responses. When the GA hormone receptor GA-INSENSITIVE DWARF1 (GID1) binds GA, it undergoes a conformational change that allows binding to the DELLA repressor. Formation of the GID1–GA–DELLA complex leads to DELLA destruction via the ubiquitin proteasome pathway, thereby lifting DELLA repression of GA responses such as seed germination and stem elongation (reviewed by Davière and Achard, Reference Davière and Achard2013). Many GA-response mutants have been described in cereals, but only a few are significantly affected in their level of dormancy. For example, the Rht (reduced height) alleles produce a GA-insensitive phenotype because the mutated DELLA protein is resistant to GID1–GA targeted degradation and constitutively represses the GA signal. This is the case for Rht3, a dominant allele in an extremely dwarf wheat cultivar (Tom Thumb): the presence of the Rht3 allele inhibits premature α-amylase production in ripening grains and enhances seed dormancy in the field (Flintham and Gale, Reference Flintham and Gale1982). Unlike mutations affecting VP1 and Sdr4, which are specific to the seed, the dominant allele Rht3 produces pleiotropic defects outside the seed, affecting plant height and flowering time. Besides this dominant DELLA allele, no other GA-signalling component has been related to natural variation for seed dormancy in a cereal species.
Hormonal control of dormancy release
So far, evidence supports a key role for the ABA/GA balance in the establishment of a dormancy programme during early grain development (Karssen and Lacka, Reference Karssen, Lacka and Bopp1986; White and Rivin, Reference White and Rivin2000; White et al., Reference White, Proebsting, Hedden and Rivin2000; Finch-Savage and Leubner-Metzger, Reference Finch-Savage and Leubner-Metzger2006). Important physiological changes take place in the grain as it approaches physiological maturity and maximum dry weight, which include programmed cell death of the starchy endosperm cells and rapid dehydration of the remaining living tissues in the embryo and the aleurone layer. After physiological maturity, dehydration proceeds until an equilibrium with ambient humidity is reached. In many cereal varieties, dormancy release begins after harvest maturity, but in others, dormancy release begins soon after physiological maturity is attained (as in several barley cultivars), or even before this (e.g. grain sorghum PHS-susceptible lines). Analysis of ABA content in the embryos of maturing grains of wheat, sorghum and barley shows a sharp decrease in ABA between physiological maturity and harvest maturity (Walker-Simmons, Reference Walker-Simmons1987; Steinbach et al., Reference Steinbach, Benech-Arnold and Sánchez1997; Benech-Arnold et al., Reference Benech-Arnold, Giallorenzi, Frank and Rodríguez1999). Nevertheless, ABA content alone does not explain dormancy release in wheat and sorghum genotypes with contrasting dormancy dynamics, and embryo sensitivity to ABA decreases precociously in short-lived dormancy grains from both species (Walker-Simmons, Reference Walker-Simmons1987; Steinbach et al., Reference Steinbach, Benech-Arnold and Sánchez1997). In barley, changes in ABA content and sensitivity to ABA occur during late maturation stages, and in this case both variables correlate with dormancy loss occurring in two cultivars with contrasting timing of dormancy release (Benech-Arnold et al., Reference Benech-Arnold, Giallorenzi, Frank and Rodríguez1999). Again, ABA appears as the key regulator of the observed patterns of dormancy release.
Although dormancy loss may begin soon after physiological maturity in some cereal varieties, others maintain high dormancy until harvest, and dormancy release takes place in the mature, dry grain as part of the afterripening process. Dry afterripening is generally effective at a seed moisture content between 5 and 18% fresh weight basis, but it can be prevented at very low moisture contents (Bazin et al., Reference Bazin, Langlade, Vincourt, Arribat, Balzergue, El-Maarouf-Bouteau and Bailly2011) or by low temperatures ( − 18°C). Transcriptional analysis of dormant (freshly harvested) and dry afterripened (non-dormant) wheat grains shows no significant changes in hormone-related transcripts (A. Liu et al., Reference Liu, Sehgal, Li, Lin, Trick, Yu, Gill and Bai2013; Chitnis et al., Reference Chitnis, Gao, Yao, Jordan, Park and Ayele2014), suggesting that changes in the transcription of hormone-related genes during dry storage are less likely to be an integral part of mechanisms underlying dormancy decay by afterripening. Endogenous hormone concentrations [ABA, jasmonic acid and indole-acetic acid (IAA)] also remain unchanged during dry afterripening of wheat grains (A. Liu et al., Reference Liu, Sehgal, Li, Lin, Trick, Yu, Gill and Bai2013). Additional studies with seeds from other species also support the observation that no relevant transcriptional changes take place during dry afterripening that can account for changes in dormancy (Meimoun et al., Reference Meimoun, Mordret, Langlade, Balzergue, Arribat, Bailly and El-Maarouf-Bouteau2014). In anhydrobiotic conditions of afterripening, non-enzymatic mechanisms are good candidates for playing a role in dormancy release. Oxidation of proteins and transcripts was studied in sunflower during seed dormancy release by Oracz et al. (Reference Oracz, Bouteau, Farrant, Cooper, Belghazi, Job, Job, Corbineau and Bailly2007) and Bazin et al. (Reference Bazin, Langlade, Vincourt, Arribat, Balzergue, El-Maarouf-Bouteau and Bailly2011). They observed that within the existing pool of mRNA stored in the mature grain a subset of transcripts was oxidized. Some of the identified transcripts were related to known ABA-signalling components, such as PP2C, MPK1 and ABC-2-type transporter, a putative ABA cellular transporter (Bazin et al., Reference Bazin, Langlade, Vincourt, Arribat, Balzergue, El-Maarouf-Bouteau and Bailly2011). This non-enzymatic mechanism may account for changes in ABA responsiveness observed during afterripening in cereal species, and future studies will hopefully shed some light on this possibility.
Hormones in the expression of dormancy in the imbibed grain
Soon after water uptake occurs and the embryo becomes imbibed, cell metabolism is activated and germination begins in a non-dormant seed. Although metabolism is also activated in a dormant, imbibed seed, the completion of germination remains blocked and enzymatic activity leading to embryo growth and reserve mobilization does not proceed because of repression by ABA. Embryos from dormant barley grains are able to synthesize and maintain a high ABA content upon imbibition under conditions at which dormancy is expressed, while in embryos from non-dormant grains this ABA is rapidly depleted (Benech-Arnold et al., Reference Benech-Arnold, Gualano, Leymarie, Côme and Corbineau2006; Millar et al., Reference Millar, Jacobsen, Ross, Helliwell, Poole, Scofield, Reid and Gubler2006). Hormone content is the result of two processes: synthesis and catabolism. A reduction in ABA content in embryos of non-dormant grains, or in those incubated under conditions at which dormancy is not expressed, can be due to a slow, or even repressed, biosynthesis; an increased inactivation rate; or to the co-ordinated action of both.
In barley, the rapid decline in ABA content observed in imbibing non-dormant (afterripened) grains is likely to be due to increased expression of HvABA8′OH1, a gene encoding an ABA catabolic enzyme, while it is not clearly related to the expression of HvNCEDs and other ABA biosynthesis genes (Millar et al., Reference Millar, Jacobsen, Ross, Helliwell, Poole, Scofield, Reid and Gubler2006; Barrero et al., Reference Barrero, Talbot, White, Jacobsen and Gubler2009). In addition to these changes in ABA metabolism, changes in ABA-signalling genes are also involved in dormancy release by afterripening (Benech-Arnold et al., Reference Benech-Arnold, Gualano, Leymarie, Côme and Corbineau2006; Millar et al., Reference Millar, Jacobsen, Ross, Helliwell, Poole, Scofield, Reid and Gubler2006; Gubler et al., Reference Gubler, Hughes, Waterhouse and Jacobsen2008; Barrero et al., Reference Barrero, Talbot, White, Jacobsen and Gubler2009).
Both hormonal and transcriptional analyses related to ABA metabolism were first carried out in whole-embryo samples (Millar et al., Reference Millar, Jacobsen, Ross, Helliwell, Poole, Scofield, Reid and Gubler2006), but more recently the major role of ABA (and the regulation of its metabolism) in dormancy was localized to the coleorhiza (Barrero et al., Reference Barrero, Talbot, White, Jacobsen and Gubler2009). The coleorhiza is a non-vascularized multicellular embryonic tissue that covers the seminal roots of grass seeds, and elongates in the early stages of imbibition before root emergence (Barrero et al., Reference Barrero, Talbot, White, Jacobsen and Gubler2009). Transcriptomic and hormonal-tissue-specific analysis of the barley grain has shown that the coleorhiza plays a major role in causing dormancy by acting as a barrier to root emergence, and that afterripening potentiates molecular changes related to ABA metabolism and sensitivity, light responses and the jasmonate biosynthetic pathway in the coleorhiza cells (Barrero et al., Reference Barrero, Talbot, White, Jacobsen and Gubler2009). Afterripening promotes ABA catabolism in the coleorhiza upon imbibition and also reduces sensitivity to ABA, and this is associated with differential regulation of ABA8′OH1 and of the HvLPP gene family and HvAIP2, respectively. Both HvLPP (Lipid Phosphate Phosphatase) and HvAIP2 are negative regulators of the ABA signalling pathway (Zhang et al., Reference Zhang, Garreton and Chua2005), and are induced in imbibed, afterripened barley grains.
Very little is known about upstream regulation of ABA metabolism associated with dormancy release in cereal grains, but recent evidence from wheat indicates that jasmonates have a role in vivo in co-ordinating ABA synthesis and catabolism in the embryos. Application of methyl jasmonate reduces dormancy and ABA content in imbibed wheat grains and this is mediated via a reduction in TaNCED1 and an increase in TaABA8′OH-1 expression (Jacobsen et al., Reference Jacobsen, Barrero, Hughes, Julkowska, Taylor, Xu and Gubler2013).
While maintenance of high ABA concentrations after imbibition is required for the expression of dormancy in the barley grain, this is not the case in some wheat and sorghum genotypes (Rodríguez et al., Reference Rodríguez, Mendiondo, Maskin, Gudesblat, Iusem and Benech-Arnold2009; A. Liu et al., Reference Liu, Sehgal, Li, Lin, Trick, Yu, Gill and Bai2013). In grain sorghum (S. bicolor), coat-imposed dormancy in two inbred lines with contrasting dormancy is not related to ABA content, which decreases similarly upon grain imbibition (Gualano et al., Reference Gualano, Carrari, Rodríguez, Pérez-Flores, Sánchez, Iusem and Benech-Arnold2007; Rodríguez et al., Reference Rodríguez, Mendiondo, Maskin, Gudesblat, Iusem and Benech-Arnold2009). In immature grains of both sorghum lines, embryo sensitivity to ABA shows a strong correlation with grain dormancy, and transcript abundance of ABA signalling genes (such as SbABI3, SbABI4, SbABI5 and SbPKABA) is induced during imbibition in the dormant line as compared to the non-dormant one. Analysis of GA metabolism genes and hormone content in these same sorghum lines (Rodríguez et al., Reference Rodríguez, Mendiondo, Cantoro, Auge, Luna, Masciarelli and Benech-Arnold2012) revealed that, in the non-dormant line, GA4 content increased during imbibition and before completion of germination, and this was related to a reduced expression of a GA 2-oxidase gene (SbGA2ox3) and lower content of GA34 (the inactive catabolite produced by oxidation of GA4) as compared to the more dormant genotype. A possible interaction between transcription factors belonging to the ABA signalling pathway (i.e. ABI4, ABI5) in the promotion of GA catabolism was tested recently in vitro for the sorghum gene SbGA2ox3. Both SbABI4 and SbABI5 proteins are able to bind to conserved ABA-response elements (ABREs) within a region of the SbGA2ox3 promoter. This possible regulation of GA catabolism through components of the ABA signalling pathway appears to be restricted to a functional sub-group (M3) of GA 2-oxidases existing only in the monocots (Cantoro et al., Reference Cantoro, Crocco, Benech-Arnold and Rodríguez2013). When ABA signalling begins to decline (as in PHS-susceptible sorghum line RedlandB2 approaching physiological maturity), GA amounts increase upon imbibition and favour the ABA/GA balance towards germination, thus producing a less-dormant phenotype.
During imbibition, sensitivity to GA synthesis inhibitors added to the incubation medium is strongest, further supporting the idea that GA metabolism can make a difference in the outcome of the hormonal balance. However, once sensitivity to ABA is low enough, germination no longer relies on GA de novo synthesis, as inferred from the lack of response to biosynthesis inhibitors in sorghum (Rodríguez et al., Reference Rodríguez, Mendiondo, Cantoro, Auge, Luna, Masciarelli and Benech-Arnold2012), but also from transcriptional evidence in barley (Barrero et al., Reference Barrero, Talbot, White, Jacobsen and Gubler2009). Changes in global gene expression and hormone content were also assessed during incubation of dormant (freshly harvested) and non-dormant (afterripened) wheat grains (A. Liu et al., Reference Liu, Sehgal, Li, Lin, Trick, Yu, Gill and Bai2013). ABA in whole wheat grains changed similarly during imbibition for both dormant and afterripened grains, while transcriptional data supported a decreased ABA signalling and an enhanced GA synthesis in the non-dormant grains.
The functional relationship between the hulls, oxygen availability, hormones and the expression of dormancy has been investigated in barley and oat. In these species, the glumellae impose dormancy by limiting oxygen diffusion into the imbibed embryo, and increases in glumella permeability to oxygen and embryo sensitivity to oxygen during afterripening contribute then to the improvement of germination. Also, embryos isolated from non-dormant grains germinate faster and are less sensitive to hypoxia than embryos isolated from dormant ones (Corbineau and Côme, Reference Corbineau and Côme1996, Reference Corbineau, Côme, Viémont and Crabbé2000; Bradford et al., Reference Bradford, Benech-Arnold, Côme and Corbineau2008). Oxygen availability to the embryo, regulated by the hull, could have additional effects on the synthesis of, and sensitivity to, ABA and GA, altering their balance of potential action to either promote or retard germination (Finch-Savage and Leubner-Metzger, Reference Finch-Savage and Leubner-Metzger2006; Bradford et al., Reference Bradford, Benech-Arnold, Côme and Corbineau2008). In both barley and oat, the glumellae play a role in metabolic regulation of the embryo (Lecat et al., Reference Lecat, Corbineau and Côme1992; Corbineau and Côme, Reference Corbineau, Côme, Nicolas, Bradford, Côme and Pritchard2003) but also affect hormone content, in particular by preventing leaching of ABA into the medium (Wang et al., Reference Wang, van der Meulen, Visser, Van Schaik, Van Duijn and de Boer1998; Jacobsen et al., Reference Jacobsen, Pearce, Poole, Pharis and Mander2002) or by interfering with ABA metabolism (Benech-Arnold et al., Reference Benech-Arnold, Gualano, Leymarie, Côme and Corbineau2006; Mendiondo et al., Reference Mendiondo, Leymarie, Farrant, Corbineau and Benech-Arnold2010). Oxygen absorption by the glumellae during the first 12–14 h after beginning of imbibition of dormant barley grains (Lenoir et al., Reference Lenoir, Corbineau and Côme1986) appears to be related to ABA metabolism in the embryo, as a transient increase in ABA content occurs within this period in dormant grains but not in afterripened ones (Benech-Arnold et al., Reference Benech-Arnold, Gualano, Leymarie, Côme and Corbineau2006). Removal of the glumellae from barley grains suppresses this initial increase in embryo ABA content during the first hours of imbibition, but this pattern can be restored by incubating naked grains (i.e. without glunellae) under hypoxia, which also blocks germination (Benech-Arnold et al., Reference Benech-Arnold, Gualano, Leymarie, Côme and Corbineau2006). In addition to a possible role of oxygen as a limiting substrate in ABA catabolism mediated by ABA8′OH in the embryo, an oxygen-sensing mechanism such as the N-end rule pathway could be involved in the control of germination in cereal grains, as has been described in Arabidopsis (Holman et al., Reference Holman, Jones, Russell, Medhurst, Ubeda Tomás, Talloji, Marquez, Schmuths, Tung, Taylor, Footitt, Bachmair, Theodoulou and Holdsworth2009; Gibbs et al., Reference Gibbs, Lee, Isa, Gramuglia, Fukao, Bassel, Correia, Corbineau, Theodoulou, Bailey-Serres and Holdsworth2011). The presence of the glumellae alters the expression of genes involved in ABA synthesis (HvNCED1 and HvNCED2), ABA catabolism (HvABA8′OH1) and signalling (HvABI5, HvVP1 and HvPKABA) with respect to de-hulled grains, but curiously this effect cannot be mimicked by artificially imposed hypoxia (Mendiondo et al., Reference Mendiondo, Leymarie, Farrant, Corbineau and Benech-Arnold2010), suggesting that the effect of the glumellae is not limited to oxygen deprivation. Moreover, although the hypoxia imposed by the glumellae increases the responsiveness of the embryo to ABA (both directly and through an altered ABA metabolism) and reduces its sensitivity to GA, other factors related to the presence of the glumellae are likely to exist that contribute to regulate hormone action in the embryo (Benech-Arnold et al., Reference Benech-Arnold, Gualano, Leymarie, Côme and Corbineau2006; Bradford et al., Reference Bradford, Benech-Arnold, Côme and Corbineau2008; Mendiondo et al., Reference Mendiondo, Leymarie, Farrant, Corbineau and Benech-Arnold2010). Future studies will help us to understand better the complex interrelationships between the oxygen availability to the embryo as regulated by the covering structures, and hormone metabolism and signalling, and their changes during dormancy expression and germination.
Integration of environmental signals through hormone action
Environmental conditions, for example soil moisture and temperature, oxygen availability, and light, regulate seed germination, dormant grains being more sensitive to external factors than non-dormant ones (see the Introduction). These environmental signals are integrated through hormone metabolism and signalling, the ABA/GA ratio having been proposed as the central component of this regulatory network (Karssen and Lacka, Reference Karssen, Lacka and Bopp1986; Finch-Savage and Leubner-Metzger, Reference Finch-Savage and Leubner-Metzger2006).
Water
Grain imbibition is associated with changes in ABA and GA content of the embryo, which depend on the cultivar and dormancy depth (see above). The expression of dormancy in barley and oat grains is associated with maintenance of a higher ABA content, while embryo ABA decreases sharply in non-dormant grains or dormant ones placed in conditions that do not allow expression of dormancy (Ried and Walker-Simmons, Reference Ried and Walker-Simmons1990; Wang et al., Reference Wang, Heimovaara-Dijkstra and Van Duijn1995; Jacobsen et al., Reference Jacobsen, Pearce, Poole, Pharis and Mander2002; Benech-Arnold et al., Reference Benech-Arnold, Gualano, Leymarie, Côme and Corbineau2006; Millar et al., Reference Millar, Jacobsen, Ross, Helliwell, Poole, Scofield, Reid and Gubler2006; Leymarie et al., Reference Leymarie, Robayo-Romero, Gendreau, Benech-Arnold and Corbineau2008; Hoang et al., Reference Hoang, Sotta, Gendreau, Bailly, Leymarie and Corbineau2013b). However, ABA metabolism is also modulated by grain moisture content. In barley, partial hydration of the grains at a moisture content lower than 0.40–0.45 g H2O (g dry weight)− 1 (which does not allow germination of either dormant or non-dormant grains) correlates with a maintenance of ABA content close to its initial value (Gendreau et al., Reference Gendreau, Romaniello, Barad, Leymarie, Benech-Arnold and Corbineau2008; Hoang et al., Reference Hoang, Sotta, Gendreau, Bailly, Leymarie and Corbineau2013b). Expression of genes involved in ABA and GA metabolism is water dependent; that of HvABA8′OH1, HvNCED1 and HvNCED2 (involved in ABA metabolism) and of HvGA2ox1, HvGA2ox3 and HvGA2ox5 (involved in GA catabolism) are not affected at moisture content as low as 0.40–0.45 g H2O (g dry weight)− 1, when transcript abundance of HvGA3ox2, HvGA20ox1 and HvGA20ox3, involved in GA synthesis, is about twofold higher than in dry grains (Hoang et al., Reference Hoang, Sotta, Gendreau, Bailly, Leymarie and Corbineau2013b).
Temperature
Responsiveness of dormant grains to temperature is strongly correlated with depth of dormancy. In temperate cereals (e.g. wheat, oat and barley) incubation at high temperatures (which enhance dormancy expression) results in the maintenance of high ABA concentrations in the embryo (Benech-Arnold et al., Reference Benech-Arnold, Gualano, Leymarie, Côme and Corbineau2006; Leymarie et al., Reference Leymarie, Robayo-Romero, Gendreau, Benech-Arnold and Corbineau2008; Hoang et al., Reference Hoang, Sotta, Gendreau, Bailly, Leymarie and Corbineau2013b). High incubation temperatures can inhibit germination of barley grains with primary dormancy, but can also induce secondary dormancy, which is evidenced after transfer to non-inhibitory conditions (e.g. 15°C for barley). Grains showing both primary and secondary dormancy display higher ABA contents during imbibition as compared to non-dormant grains (Hoang et al., Reference Hoang, Sotta, Gendreau, Bailly, Leymarie and Corbineau2013b). While the maintenance of higher ABA content during the expression of primary dormancy at 30°C is mainly correlated with reduced expression of an ABA catabolism gene, HvABA8′OH1, the induction of secondary dormancy by incubation at 30°C is associated with an increase in the expression of ABA biosynthesis genes HvNCED1 and HvNCED2 (Hoang et al., Reference Hoang, Sotta, Gendreau, Bailly, Leymarie and Corbineau2013b). In contrast, the decrease in ABA content observed at low incubation temperatures (at which primary dormancy is not expressed) is associated with an increase in HvABA8′OH1 transcript abundance, but with no significant change in the expression of HvNCED1 and HvNCED2, suggesting a key role of ABA catabolism (Leymarie et al., Reference Leymarie, Robayo-Romero, Gendreau, Benech-Arnold and Corbineau2008; Hoang et al., Reference Hoang, Sotta, Gendreau, Bailly, Leymarie and Corbineau2013b). In parallel, exposure of imbibed barley grains to high temperatures that induce secondary dormancy, also affect the relative expression of genes involved in GA synthesis and catabolism. In particular, expression of HvGA3ox2, a gene involved in GA biosynthesis, is stronger at 15°C than at 30°C, while expression of HvGA2ox3, a gene involved in GA catabolism, is enhanced at 30°C as compared to at 15°C (Hoang et al., Reference Hoang, Sotta, Gendreau, Bailly, Leymarie and Corbineau2013b). In consequence, the HvGA3ox2/ HvGA2ox3 transcript abundance ratio is higher at 15°C (8.8) than at 30°C (0.32) in grains with primary dormancy, and remains low at 15°C in grains with secondary dormancy.
Incubation at high temperature also results in an increase in embryo sensitivity to ABA (Walker-Simmons, Reference Walker-Simmons1988; Corbineau et al., Reference Corbineau, Poljakoff-Mayber and Côme1991, Reference Corbineau, Black and Côme1993; Benech-Arnold et al., Reference Benech-Arnold, Gualano, Leymarie, Côme and Corbineau2006; Leymarie et al., Reference Leymarie, Robayo-Romero, Gendreau, Benech-Arnold and Corbineau2008). In contrast, in sorghum (a summer crop, dormancy expression being promoted at lower temperatures), the improvement of germination at high temperatures cannot be explained by changes in either embryo ABA sensitivity or ABA content (Benech-Arnold et al., Reference Benech-Arnold, Enciso, Sanchez and Rodriguez2003).
Recent genetic evidence has identified the wheat homologue of MOTHER OF FT and TF1 (TaMFT) as a key regulator of germination temperature responses in dormant wheat (Nakamura et al., Reference Nakamura, Abe, Kawahigashi, Nakazono, Tagiri, Matsumoto, Utsugi, Ogawa, Handa, Ishida, Mori, Kawaura, Ogihara and Miura2011). Low maturation (dormancy promoting) temperatures result in higher expression of TaMFT in the embryo at maturity. It is interesting to note that, in contrast to the role of TaMFT in wheat grains, AtMFT is a negative regulator of ABA sensitivity during Arabidopsis seed germination (Xi et al., Reference Xi, Liu, Hou and Yu2010).
Oxygen
Hypoxia imposed on the embryo results in changes in the expression of various genes involved in ABA and GA metabolism and affects the embryo sensitivity to these hormones (Benech-Arnold et al., Reference Benech-Arnold, Gualano, Leymarie, Côme and Corbineau2006; Bradford et al., Reference Bradford, Benech-Arnold, Côme and Corbineau2008; Mendiondo et al., Reference Mendiondo, Leymarie, Farrant, Corbineau and Benech-Arnold2010; Hoang et al., Reference Hoang, Bailly, Corbineau and Leymarie2013a). During imbibition, embryo ABA content decreases more slowly in hypoxia than in air either at 15 or 30°C, but this maintenance of ABA content is not associated with a down-regulation of HvABA8’OH1 expression (Mendiondo et al., Reference Mendiondo, Leymarie, Farrant, Corbineau and Benech-Arnold2010; Hoang et al., Reference Hoang, Bailly, Corbineau and Leymarie2013a). At 30°C, hypoxia (with a maximum effect at 3–5% oxygen) induces the expression of HvABA8′OH1 at least during the first 14 h of imbibition (Mendiondo et al., Reference Mendiondo, Leymarie, Farrant, Corbineau and Benech-Arnold2010), while at 15°C, it does not affect the expression of ABA metabolism genes after the first day (Hoang et al., Reference Hoang, Bailly, Corbineau and Leymarie2013a). Expression of GA metabolism genes is strongly regulated by hypoxia after 1 d at 15°C, with a strong promotion of HvGA2ox3 (64-fold compared with grains incubated in air) and inhibition of HvGA3ox2 and HvGA20ox1, suggesting that hypoxia reduces GA content (Hoang et al., Reference Hoang, Bailly, Corbineau and Leymarie2013a).
In addition, embryo sensitivity to ABA increases by several orders of magnitude in hypoxia and seeds become unresponsive to GA at low oxygen (Benech- Arnold et al., Reference Benech-Arnold, Gualano, Leymarie, Côme and Corbineau2006; Bradford et al., Reference Bradford, Benech-Arnold, Côme and Corbineau2008). At 20°C, for example, GA sensitivity of barley grains decreases by six orders of magnitude between 21% and 10% oxygen (Bradford et al., Reference Bradford, Benech-Arnold, Côme and Corbineau2008).
Light
The inhibitory action of white or blue light on the germination of barley and wheat grains is associated with an increase in embryo ABA content, resulting in an up-regulation of NCED1 and NCED2 and a down-regulation of ABA8′OH-1 (Jacobsen et al., Reference Jacobsen, Pearce, Poole, Pharis and Mander2002, Reference Jacobsen, Barrero, Hughes, Julkowska, Taylor, Xu and Gubler2013; Gubler et al., Reference Gubler, Hughes, Waterhouse and Jacobsen2008; Hoang et al., Reference Hoang, Sechet, Bailly, Leymarie and Corbineau2014), and with an increase in embryo sensitivity to ABA (Hoang et al., Reference Hoang, Sechet, Bailly, Leymarie and Corbineau2014). In barley, blue light also inhibits the expression of HvGA3ox2, improves that of HvGA2ox3 and HvGA2ox5, leading to reduced GA content and signalling (Gubler et al., Reference Gubler, Hughes, Waterhouse and Jacobsen2008; Hoang et al., Reference Hoang, Sechet, Bailly, Leymarie and Corbineau2014). As in Arabidopsis (Seo et al., Reference Seo, Hanada, Kuwahara, Endo, Okamoto, Yamauchi, North, Marion-Poll, Sun, Koshiba, Kamiya, Yamaguchi and Nambara2006), the down-regulation of HvGA3ox2 by blue light might result from the increase in ABA content. Results obtained by Barrero et al. (Reference Barrero, Downie, Xu and Gubler2014) using transgenic lines down-regulating genes encoding the blue light receptor cryptochrome (HvCRY1 and HvCRY2), and the partial reversion of the inhibitory effect of blue light by green light (Hoang et al., Reference Hoang, Sechet, Bailly, Leymarie and Corbineau2014), demonstrate that the hormonal regulation by blue light is mediated by cryptochrome CRY1 photoreceptor. The blue light response has been suggested to play a significant ecophysiological role, mediating germination responses based on the extent of exposure of the grain to light (Barrero et al., Reference Barrero, Jacobsen, Talbot, White, Swain, Garvin and Gubler2012). Grains that are on the surface of the soil will be exposed to more blue light and thus are less likely to germinate than those that are buried deeper in the soil.
So far the evidence indicates that only members of the grass family respond to the blue portion of the spectrum in germination assays. This is in contrast to dicot species, for which there is strong evidence for phytochrome-mediated regulation of germination, and blue light appears to have no effects on imbibing seeds (Barrero et al., Reference Barrero, Downie, Xu and Gubler2014) or are restricted to imbibition conditions during which dormancy is strongly expressed, as reported for lettuce (Small et al., Reference Small, Spruit, Blaauw-Jansen and Blaauw1979). In a recent study of the wild grass Brachypodium distachyon, red–far red reversibility of germination of dormant grains was demonstrated, indicating that phytochrome B control of germination exists in a grass that is closely related to the cereals (Barrero et al., Reference Barrero, Jacobsen, Talbot, White, Swain, Garvin and Gubler2012). It has been speculated that red and far red effects have been selected against during domestication of cereals to improve uniformity of germination.
Practical implications of knowledge about dormancy in cereals
Pre-harvest sprouting in cereals
Dormancy is an important agronomic trait for many cereal crop species and a number of molecular strategies are now being pursued to manipulate the expression of dormancy to protect grain quality and yield. In cereal-growing regions of the world where there is a high probability of rain at harvest time, PHS is a major constraint to the production of high-quality grains (Derera, Reference Derera and Derrera1989; Gubler et al., Reference Gubler, Millar and Jacobsen2005; Black et al., Reference Black, Bewley and Halmer2006; Kulwal et al., Reference Kulwal, Mir, Kumar and Gupta2010). PHS can be a serious agronomical problem in temperate or tropical areas (Meredith and Pomeranz, Reference Meredith, Pomeranz and Pomeranz1985), for wheat (King, Reference King, Kruger and Laberge1983), rice (Maruyama, Reference Maruyama1980; Juliano and Chang, Reference Juliano, Chang and Mares1987), maize (Neill et al., Reference Neill, Horgan and Rees1987) and sorghum (Clark et al., Reference Clark, Collier and Langston1967; Maiti et al., Reference Maiti, Raju and Bidinger1985), depending on the weather conditions. The problem is present in all major winter cereal crops, such as wheat, where it is estimated to cause losses of up to US$1 billion worldwide (Black et al., Reference Black, Bewley and Halmer2006) and it is also a serious problem in tropical and subtropical crops, such as rice and sorghum. It is routinely associated with two phenotypes in the harvested grains: high α-amylase activity (generally measured by falling number) and the presence of sprouted grains (Mares, Reference Mares and Derrera1989). Following rain, germination is initiated rapidly in PHS-susceptible grains, triggering expression of aleurone hydrolytic enzymes such as α-amylases and proteases, which then, in turn, degrade the stored starch and protein reserves in the starchy endosperm. Once endosperm degradation is initiated, then the usefulness of the grains for commercial processes, such as bread and beer making, declines rapidly. Grains with severe PHS damage often are downgraded to animal feed, resulting in major losses in revenue to farmers. Ultimately the sprouting damage can lead to loss of longevity or grain viability following grain desiccation after harvest (Gualano et al., 2014). It is now clear from mapping of PHS susceptibility and dormancy quantitative trait loci (QTLs) that many genes co-locate to the same chromosomal regions, providing strong support for the contention that the prevalence of PHS in cereals is predominantly due to the lack of dormancy at harvest ripeness (Anderson et al., Reference Anderson, Sorells and Tanksley1993; Mares and Mrva, Reference Mares and Mrva2001; Ogbonnaya et al., Reference Ogbonnaya, Imtiaz, Ye, Hearnden, Hernandez, Eastwood, van Ginkel, Shorter and Winchester2008). Earlier studies suggesting that ear morphology and composition also impact PHS remain to be confirmed (King, Reference King and Derrera1989). It is now recognized that moderate to high levels of grain dormancy are required for protection against PHS, but in certain instances this may cause problems for some downstream applications. In the case of malting barley, rapid and uniform germination is required for the production of high-quality malts (Hicky et al., Reference Hicky, Lawson, Arief, Fox, Frankowiak and Dieters2012; Edney et al., Reference Edney, Legge, Izydorczyk, Demeke and Rossnagel2013). Barley grains that retain any residual dormancy at harvest will need to be afterripened to eliminate dormancy before malting, resulting in extra storage costs.
Due to the worldwide prevalence of PHS in cereals there has been considerable research and breeding effort directed towards identifying genetic solutions to increase dormancy in grains at maturity. In breeding operations, grain dormancy is typically measured by germination tests of harvest-ripe grain, and is generally restricted to advanced lines late in the breeding cycle due to the time-consuming nature of the phenotypic screen. Marker-assisted selection of desirable dormancy genes will overcome this bottleneck and provide a strategy for early generation selection to enrich advanced generations with a suitable combination of dormancy alleles.
Genetic analysis of grain dormancy of cereals reveals that it is a complex trait controlled by multiple genes that are spread across multiple chromosomal locations. Genomic tools such as QTL mapping in combination with map-based cloning have been used with success in both rice and wheat, to discover novel genes/alleles that can be used by breeders for marker-assisted introgression. Recently, other approaches, such as genome-wide-association studies, have been used to identify DNA markers linked to high-dormancy alleles (Jaiswal et al., Reference Jaiswal, Mir, Mohan, Balyan and Gupta2012; Kuwal et al., Reference Kuwal, Ishikawa, Benscher, Feng, Yu, Jadhav, Mehetre and Sorrells2012; Rehman Arif et al., Reference Rehman Arif, Neumann, Nagel, Kobiljski, Lohwasser and Borner2012; Rathi et al., Reference Rathi, Pathak, Yadav, Kumar and Sama2014). Similarly transformation and mutation breeding also offer considerable promise as strategies to eliminate PHS in cereals. The next section will focus on the progress towards eliminating PHS in wheat and rice.
Molecular breeding strategies to eliminate PHS in wheat and rice
Many QTL studies have been performed in rice and wheat to identify chromosomal regions that contribute to natural variation in dormancy. In general, the strategy used has been to develop biparental populations derived from parental cultivars that differ in dormancy, and in some cases recombinant inbred lines have also been developed to assist with the mapping. In rice, many dormancy-related QTLs have been identified with prominent QTLs located on chromosome 3 (Sdr1), chromosome 12 (qSD12) and chromosome 7 (Sdr4) (Lin et al., Reference Lin, Sasaki and Yano1998; Takeuchi et al., Reference Takeuchi, Lin, Sasaki and Yano2003; Gu et al., Reference Gu, Kianian and Foley2004; Gao et al., Reference Gao, Ren, Lu, Sin, Li, Gao, Lou, Yan and Zhang2008; Hori et al., Reference Hori, Sugimoto, Nonoue, Ono, Matsubara, Yamanouchi, Abe, Takeuchi and Yano2010; Lu et al., Reference Lu, Xie, Yang, Wang, Liu, Zhang, Jiang and Wan2011; Wang et al., Reference Wang, Cheng, Lai, Du, Huang, Wang and Zhang2014). Similarly, in wheat many QTLs have been identified, with the major ones located on chromosome 4A (Kato et al., Reference Kato, Nakamura, Tabiki and Miura2001; Mares and Mrva, Reference Mares and Mrva2001; Flintham et al., Reference Flintham, Adlam, Bassoi, Holdsworth and Gale2002; Mares et al., Reference Mares, Mrva, Cheong, Williams, Watson, Storlie, Sutherland and Zou2005; Mori et al., Reference Mori, Uchino, Chono, Kato and Miura2005; Chen et al., Reference Chen, Cai and Bai2008; Torada et al., Reference Torada, Koike, Ikeguchi and Tsutsui2008; Singh et al., Reference Singh, Matus-Cadiz, Baga, Hucl and Chibbar2010; Kuwal et al., Reference Kuwal, Ishikawa, Benscher, Feng, Yu, Jadhav, Mehetre and Sorrells2012) and group 3 chromosomes (Groos et al., Reference Groos, Gay, Perretant, Gervais, Bernard, Dedryver and Charmet2002; Osa et al., Reference Osa, Kato, Mori, Shindo, Torada and Miura2003; Kulwal et al., Reference Kulwal, Kumar, Gaur, Khurana, Khurana, Tyagi, Balyan and Gupta2005; Mori et al., Reference Mori, Uchino, Chono, Kato and Miura2005; Imtiaz et al., Reference Imtiaz, Ogbonnaya, Oman and Van Ginkel2008; Liu et al., Reference Liu, Cai, Graybosch, Chen and Bai2008; Fofana et al., Reference Fofana, Humphreys, Rasul, Cloutier, Brule-Babel, Woods, Lukow and Somers2009; Kumar et al., Reference Kumar, Kumar, Singh, Garg, Chuneja, Balyan and Gupta2009; Mares et al., Reference Mares, Rathjen, Mrva and Cheong2009; S. Liu et al., Reference Liu, Sehgal, Li, Lin, Trick, Yu, Gill and Bai2013).
The Sdr4 QTL in rice is a major contributor to dormancy in indica cultivars (Lin et al., Reference Lin, Sasaki and Yano1998). Molecular cloning of the Sdr4 allele from japonica and indica cultivars shows that it encodes a novel nuclear protein of unknown function that is under the control of OsVP1 (Sugimoto et al., Reference Sugimoto, Takeuchi, Ebana, Miyao, Hirochika, Hara, Ishiyama, Kobayashi, Ban, Hattori and Yano2010). The dormant indica allele identified in Kasalath rice appears to be widely distributed in the indica group and in a wild rice ancestor Oryza rufipogon. Interestingly, analysis of the Ossdr4 mutant revealed that several germination-related genes, such as OsGA20ox-1, are down-regulated in the mutant grains compared to wild type. Isolation of TaSdr4 orthologues on the wheat chromosome 2A, B and D genomes revealed allelic variants for TaSdr-B1(a and b) between high- and low-dormancy Chinese wheat lines (Zhang et al., Reference Zhang, Miao, Xia and He2014). Linkage and association mapping of the alleles showed high correlation of the TaSdr1-B1b allele with low-dormancy trait compared to the TaSdr-B1a allele. Analysis of the geographic distribution of the TaSdr-B1a allele revealed that it was present in higher frequencies in Chinese, Japanese, Australian and Argentine cultivars from PHS-susceptible regions, but not European cultivars.
Compared to rice, map-based cloning of candidate genes in wheat is far more difficult because of its hexaploid genome and the absence of a quality genome sequence. The major QTL for grain dormancy in wheat, which is on chromosome 4AL, explains up to 40% of the phenotypic variability in some populations. The gene underlying the QTL has not been identified but it is thought to be syntenic with the barley SD2 dormancy QTL (Li et al., Reference Li, Tarr, Lance, Harasymow, Uhlmann, Westcot, Young, Grime, Cakir, Broughton and Appels2003) on chromosome 5HL (Flintham et al., Reference Flintham, Adlam, Bassoi, Holdsworth and Gale2002; Zhang et al., Reference Zhang, Li, Tay, Lance, Mares, Cheong, Cakir, Ma and Appels2008). The cloning of this major QTL will be an important step in providing wheat and barley breeders with new opportunities to increase dormancy in new cultivars. Progress has been made largely through the candidate gene approach, such as that described above for TaSdr. The discovery that TaMFT has a role in mediating the effect of temperature on dormancy acquisition during grain development led researchers to investigate whether allelic variation may be responsible for dormancy differences in two wheat cultivars that show differential germination profiles when grown at low temperatures (Nakamura et al., Reference Nakamura, Abe, Kawahigashi, Nakazono, Tagiri, Matsumoto, Utsugi, Ogawa, Handa, Ishida, Mori, Kawaura, Ogihara and Miura2011). Low-resolution mapping in recombinant inbred lines generated between the highly dormant Zen cultivar and the less dormant Chinese Spring cultivar indicates that TaMFT is located in a QTL region on chromosome 3A. Analysis of TaMFT expression identified differences in expression of the two parental alleles during grain development, with the Zen allele being more highly expressed than the Chinese Spring allele during the latter stages of grain maturation. These results are consistent with evidence from functional testing in wheat embryos, which shows that increased TaMFT expression is associated with increased dormancy. It is speculated that the differential expression between the two alleles is due to single nucleotide polymorphisms (SNPs) in the TaMFT promoter sequences, which may affect a bZIP binding site. The earlier finding is supported by fine mapping of a QTL on chromosome 3AS (Qphs.pseru-3AS) in a population derived from a PHS-resistant wheat cultivar (Rio Blanco) and PHS- susceptible cultivar NW97S186 (S. Liu et al., Reference Liu, Sehgal, Li, Lin, Trick, Yu, Gill and Bai2013). Physical mapping using a minimum bacterial artificial chromosome (BAC) tiling path narrowed the QTL region to a 201-kb contig which contains six genes, including TaMFT which, based on gene expression profiles in imbibed grains, is proposed to be the most likely candidate gene. Functional proof was provided by showing that RNA interference (RNAi) suppression of TaMFT results in increased germination of grain in sprouting assays. Molecular analysis of the alleles showed that increased PHS susceptibility associated with the NW97A186 allele is due to SNPs that cause mis-splicing of the gene, resulting in a loss of the functional allele. The discovery of multiple haplotypes of the TaMFT gene provides breeders with a choice of alleles to breed for PHS resistance.
As described above, red-grained wheat and rice usually show higher PHS resistance than white-grained cultivars (Cohn and Hughes, Reference Cohn and Hughes1981; Flintham, Reference Flintham2000). The association between pericarp colour and dormancy in wheat has provided breeders with an easy phenotypic screen to select cultivars that have higher dormancy. The selection for dormant red-grained cultivars has been particularly strong in wheat-growing regions of North America, Europe and China, but other countries such as Australia have preferred to select for white-grained wheats with moderate dormancy. In mapping populations between white- and red-grained wheat cultivars, the grain colour is controlled by dominant R-1 genes encoded by Tamyb10 located on chromosomes 3AL, 3BL and 3DL (Himi and Noda, Reference Himi and Noda2005; Himi et al., Reference Himi, Maekawa, Miura and Noda2011). Analysis of white and red grains suggests that the Tamyb10 gene regulates the early steps in the flavonoid biosynthetic pathway in the coat during grain development. However, it is important to note that the linkage between red-grained wheats and dormancy is not tight, since it has been found that the expression of the R-1 genes on their own are not sufficient to provide dormancy but requires epistatic interactions with other dormancy QTLs (Mares et al., Reference Mares, Mrva, Cheong, Williams, Watson, Storlie, Sutherland and Zou2005). Analysis of white mutants of red-grained wheats indicates that the red gene contribution to dormancy is small but significant (Warner et al., Reference Warner, Kudrna, Spaeth and Jones2000; Himi et al., Reference Himi, Mares, Yanagisawa and Noda2002).
In contrast to wheat, there has been strong selection against the red pericarp colour during rice domestication, due to visual and flavour characteristics associated with the red pigments (Sweeney et al., Reference Sweeney, Thompson, Cho, Park, Williamson, Bustmante and McCouch2007). Genetic analysis has identified two loci that control red grain colour in rice, with one locus encoding a basic helix–loop–helix transcription factor that results in increased dormancy when introduced in white-grained rice (Sweeney et al., Reference Sweeney, Thomson, Pfeil and McCouch2006; Gu et al., Reference Gu, Foley, Horvath, Anderson, Feng, Zhang, Mowry, Ye, Suttle, Kadowaki and Chen2011).
In parallel to traditional breeding efforts to increase dormancy, molecular strategies such as transgenic, forward and reverse genetic approaches have also been used to develop new wheat germplasm with increased dormancy. However, the difficulty in mutagenic screens in wheat is compounded by the presence of three genomes, thus often requiring recessive mutations in two or three homeologous genes to reveal a phenotype. Dominant mutations only require an alteration in one of the homeologous genes to generate a phentoype. One successful approach using these methods has been to manipulate ABA catabolism and signalling in wheat grains to increase dormancy. A double mutant carrying a deletion in the ABA 8′ hydroxylase-1 gene in the A genome and an insertion the homeologous gene in the D genome results in reduced ABA catabolism and increased dormancy compared to wild-type grains (Chono et al., Reference Chono, Matsunaka, Seki, Fujita, Kiribuchi-Otabe, Oda, Kojima, Kobayashi and Kawakami2013). Further work will be required to assess the performance of the double mutant lines exposed to abiotic stresses under field conditions (Ji et al., Reference Ji, Dong, Shiran, Talbot, Edlington, Hughes, White, Gubler and Dolferus2011). Mis-splicing of the wheat Viviparous-1 (TaVp1) transcription factor involved in ABA signalling has been suggested as a contributor to PHS susceptibility in wheat (McKibbin et al., Reference McKibbin, Wilkinson, Bailey, Flintham, Andrew, Lazzeri, Gale, Lentone and Holdsworth2002; Wilkinson et al., Reference Wilkinson, Lenton and Holdsworth2005). To overcome this putative defect, VP1 genes from wild oats (McKibbin et al., Reference McKibbin, Wilkinson, Bailey, Flintham, Andrew, Lazzeri, Gale, Lentone and Holdsworth2002) and maize (Huang et al., Reference Huang, Qu, Li, Zuo, Zhao and Liao2012) have been over-expressed in transgenic wheat, resulting in increased dormancy and PHS resistance. Identification of further targets for manipulation have been found by identifying wheat genes that are orthologous to characterized dormancy genes in other plants. The demonstration that overexpression of wheat Delay of Germination 1 in transgenic wheat grains increases dormancy opens up new avenues to prevent PHS (Ashkawa et al., Reference Ashkawa, Mori, Nakamura and Abe2014). A novel transgenic strategy to enhance ABA biosynthesis using positive feedback has been proposed as an alternative solution to PHS in cereals (Nonogaki et al., Reference Nonogaki, Sali, Nambara and Nonogaki2014). The strategy, which has been demonstrated in a model plant, is based on the expression of a chimeric gene consisting of an ABA-responsive promoter fused to an ABA biosynthetic gene that is predicted to result in a large increase in ABA content in the cereal grain.
Forward mutant screens have been performed in wheat cultivars to identify mutants with increased dormancy (Kobayashi et al., Reference Kobayashi, Takumi and Nakamura2008; Schramm et al., Reference Schramm, Abellara, Strader, Campbell and Steber2010, Reference Schramm, Nelson, Kidwell and Steber2013). Germination screens with mutagenized white grains that have been afterripened for 6 months identified three ABA hypersensitive mutants (Schramm et al., Reference Schramm, Nelson, Kidwell and Steber2013). All three lines with increased sensitivity to ABA have higher grain dormancy. The hypersensitive mutants display a slower loss of dormancy during afterripening compared to wild-type grains but, interestingly, the increased sensitivity to ABA is still present in fully afterripened grains. One mutant was identified as being particularly useful in breeding for PHS resistance in white wheat varieties, and molecular markers linked to the mutant allele will be developed to assist this strategy.