Numerous epidemiological studies have shown an inverse association between fruit and vegetable consumption and chronic diseases, including different types of cancer and CVD( Reference Yahia 1 ). Therefore, the interest in the health benefits of fruit and vegetable consumption has increased during the last decades. Moreover, the interest in the types, concentrations and modes of action of the different components from fruits and vegetables responsible for the beneficial health effects has increased as well.
Carotenoids are natural fat-soluble pigments from plants such as orange fruits, red or yellow fruits and vegetables, and some dark green vegetables. The key property of carotenoids is their capacity for quenching singlet oxygen and free radicals depending on the number of conjugated double bonds( Reference Roldan-Gutierrez and Dolores Luque de Castro 2 ). Lycopene is an acyclic isomer of β-carotene containing linearly arranged eleven conjugated and two unconjugated double bonds( 3 ). The unique chemical properties of lycopene derive from its structure, which makes it extremely hydrophobic and soluble in tissues, milk and organic solvents( Reference Roldan-Gutierrez and Dolores Luque de Castro 2 ).
Lycopene from natural plant sources exists predominantly in an all-trans configuration, the most thermodynamically stable form with eleven carbon–carbon double bonds( Reference Clinton 4 ). Of these eleven double bonds, seven can be isomerized from the trans form to the mono- or poly-cis forms under the influence of excess heat, light or certain chemical reactions. Although the predominant form of lycopene in foods is all-trans, human blood and tissues contain mainly cis-isomers. The bioavailability of cis-lycopene was found to be higher than that of trans-lycopene and cis-lycopene is preferentially absorbed( Reference Boileau, Boileau and Erdman 5 ).
Since lycopene lacks the β-ionone ring structure, it cannot form vitamin A. Its biological effects in man have therefore been attributed to mechanisms other than vitamin A including antioxidant effects and immune response modulators( Reference Roldan-Gutierrez and Dolores Luque de Castro 2 ). Lycopene is one of the most potent antioxidants, with a singlet-oxygen-quenching ability twice as high as that of β-carotene and ten times higher than that of α-tocopherol( Reference Di, Kaiser and Sies 6 ). It has been suggested that dietary intake of tomatoes and tomato products containing lycopene might be associated with a decreased risk of chronic diseases such as cancer and CVD( Reference Mordente, Guantario and Meucci 7 – Reference Barber and Barber 11 ). However, a large-scale study did not find an association between lycopene intake and prostate cancer risk( Reference Kirsh, Mayne and Peters 12 ). In addition, a systematic review concluded that, given the limited number of randomized controlled trials published, it is not possible to support or refute the use of lycopene for the prevention or treatment of prostate cancer( Reference Ilic and Misso 13 ). It has also been suggested that nutrients with redox modulator properties, such as lycopene, might have beneficial effects on the risk of other chronic diseases such as diabetes, neurodegenerative diseases and ocular disorders, as well as on asthma and viral infections( Reference Rao and Rao 14 ).
Dietary lycopene is obtained from a limited list of foods only, in contrast to the other major carotenoids. The principal source of dietary lycopene is tomato in most people's diets. It is worth mentioning that the lycopene content varies substantially in different varieties of tomato, in different climates and with the use of different agricultural practices. Besides tomatoes and tomato-derived products (tomato juice, sauces, paste, etc.), watermelon, pink grapefruit, guava and papaya have been shown to be important sources of lycopene( Reference Rao and Rao 14 , Reference Garcia-Closas, Berenguer and Jose 15 ). Although comparative bioavailability values for lycopene from different tomato products are unknown, lycopene from processed tomato products appears to be more bioavailable than that from raw tomatoes( Reference Boileau, Boileau and Erdman 5 , Reference Unlu, Bohn and Francis 16 ). The release of lycopene from the food matrix due to processing has been reported to enhance lycopene bioavailability. Moreover, lycopene is a fat-soluble compound, which implies that absorption is improved when fat is present, and it should therefore be ingested within a mixed meal( Reference Gartner, Stahl and Sies 17 ). It has also been reported that the bioavailability of lycopene is affected by the dosage and the presence of other carotenoids, among which β-carotene. It was shown that the bioavailability of lycopene was significantly higher when it was ingested along with β-carotene than when ingested separately( Reference Agarwal and Rao 18 ).
Results from dietary surveys show mean usual intakes of lycopene from natural dietary sources in different populations between 0·5 and 5 mg/d, with high intakes up to about 8 mg/d( 19 ). High consumption of fruits and vegetables, especially tomato products, may result in occasional intakes of 20 mg lycopene/d or more( 3 ). The European Food Safety Authority's Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Foods established an Acceptable Daily Intake (ADI) for lycopene in 2008 of 0·5 mg/kg body weight (BW) per d( 19 ). This ADI value refers to lycopene from all sources, since lycopene is also reported to be used as a food colorant and ingredient in novel foods.
The aim of the present study was to assess the distribution of lycopene intake in the Belgian adult population and to determine which foods are the main contributors to the intake. Both total and cis-lycopene intakes were assessed.
Materials and methods
Food consumption data
Consumption data from the Belgian Food Consumption Survey (BFCS; dating from 2004) were used to perform the intake assessment. The target population of the BFCS comprised Belgian adults aged 15 years and older. Aims, design and methods of this survey can be found elsewhere( Reference De Vriese, Debacker and de Henauw 20 ). The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the medical ethical committee of the Scientific Institute of Public Health. Written informed consent was obtained from all participants. The sample included 3245 individuals who were randomly selected from the National Register using a multistage stratified procedure. Information on dietary intake was collected by two non-consecutive 24 h recalls. During the 24 h recall interviews, the respondent reported the quantity of all foods and beverages consumed during the preceding day. The survey covered one full year and the response rate was on average 41 %.
Food label survey
According to the EU Directive 94/36/EC on colours for use in foods, lycopene (E160d) is allowed to be used in certain categories of foods such those as listed in Annex V part 2 of the Directive( 21 ). Therefore, a label survey was performed to investigate whether food products on the Belgian market contained lycopene added as colour. The label survey was performed based on the presumption that labelling of food additives is mandatory. The labels of foods from five major Belgian supermarkets were screened for lycopene or E160d between May and August 2011.
Lycopene concentration of foods
Food samples were purchased in supermarkets and local stores. The ingredient lists of purchased foods were checked and all foods that had products containing lycopene among the ingredients listed were analysed.
A total of 322 food samples were selected, which were finely ground and homogenized before analysis. For the determination of total and cis-lycopene content in foods, an HPLC-UV method was used as previously described( Reference Cucu, Huvaere and Van Den Berghe 22 ).
Intake assessment
Both 24 h recalls, which were carried out using the EPIC-SOFT software( Reference Slimani and Valsta 23 ), were completed by 3083 participants; 1546 men and 1537 women (hence 6166 recalls).The individual intake of lycopene from a certain food product was calculated using the equation:
where Yi is the intake of the lycopene by individual i from a particular food on an interview day (mg/kg BW per d); ci is the concentration of the lycopene in the food (mg/kg); xi is the consumption of a certain food by individual i (kg); and BW i is the self-reported BW of individual i (kg). To estimate the total intake of lycopene/food group per d, individual daily intakes of lycopene from different foods were added up. The arithmetic means of the levels of lycopene in tomatoes, tomato products and foods containing lycopene derived from fruits and vegetables were calculated.
The usual intake distribution for lycopene was estimated with the Nusser method( Reference Nusser, Carriquiry and Dodd 24 ) using the C-SIDE software( 25 ). This statistical procedure adjusts for within-person or day-to-day variability. The usual intake distribution was weighted and adjusted for the age and sex distribution of the Belgian population and adjusted for day of the week and season.
In order to assess the contribution of several foods to lycopene intake, foods were classified into the following groups: tomatoes and tomato products (raw tomatoes, tomato concentrate, tomato paste, chopped/canned tomatoes); fruits and fruit products (watermelon, papaya, grapefruit, fruit salads); dairy products (cheeses with herbs, yoghurts with fruits); cereals and cereal products (chips, salted biscuits, breakfast cereals, raw pasta); biscuits; meat salads and products (meat salads, tête pressée and sausages); fish salads and products (fish salads and fish products in tomato sauce); butter with herbs; confectionery (jam, candies containing fruits); tomato and fruit juices; soups; and a group of sauces (such as ketchup, pesto rosso, pasta sauces, other sauces, tapenades) together with ready-to-eat meals containing tomatoes (such as pasta, pizza, lasagne).
The population proportion formula was used to determine the percentage contribution of each food group to the intake of lycopene( Reference Krebs-Smith, Kott and Guenther 26 ), which includes summing the amount of the component provided by the food for all individuals and dividing by the total intake of that component from all foods for the entire study population.
Results
The label study performed on the Belgian market (represented by the five main supermarkets) indicated that no lycopene as either a food colour or a novel food ingredient was used in foods. Taking into account this observation, only the intake of lycopene from natural sources was further considered in the present study.
Table 1 shows the cis- and total lycopene concentration levels in different foods. As expected, the foods containing the highest lycopene level were tomatoes and tomato-derived products such as juices, paste, concentrate and sauces. However, high variability in the lycopene content was observed for all analysed food products. In the case of fresh tomatoes for example, total lycopene content ranged from 33·3 to 103·7 mg/kg. The concentration of cis-lycopene varied between the food groups as well. As expected, highly processed foods (sauces, soups, ready-to-eat meals) contained the highest amounts of cis-lycopene compared with non-processed foods (raw tomatoes and fruits).
Table 1 Cis- and total lycopene contents of some common foods on the Belgian market
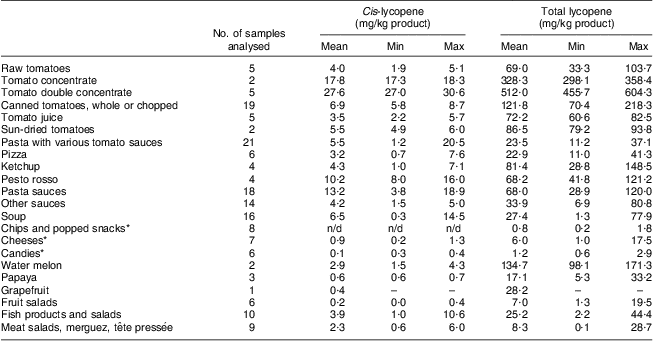
n/d, not detected.
*Only samples with ‘tomato extract’ or ‘tomato powder’ mentioned on the label were considered.
The mean total lycopene intake of the general population (n 3083) was 4·1 (sd 2·3) mg/d and 8·5 mg/d at the 95th percentile (Table 2). The total lycopene intake of men was significantly higher than that of women (4·6 (sd 2·6) mg/d on average v. 3·6 (sd 2·1) mg/d on average respectively; P < 0·001). For men the intake at the 95th percentile was 9·5 mg/d while for women the lycopene intake at the 95th percentile was 7·6 mg/d. The higher intake among men compared with women was mainly due to the higher lycopene intake via sauces and ready-to-eat meals among men (1·75 mg/d) compared with women (1·17 mg/d). For both men and women, the total lycopene intake was lower in the older than among the younger age groups.
Table 2 Estimated total lycopene intake from natural sources (in mg/kg BW per d and mg/d) of the Belgian adult population; Belgian Food Consumption Survey (BFCS), 2004
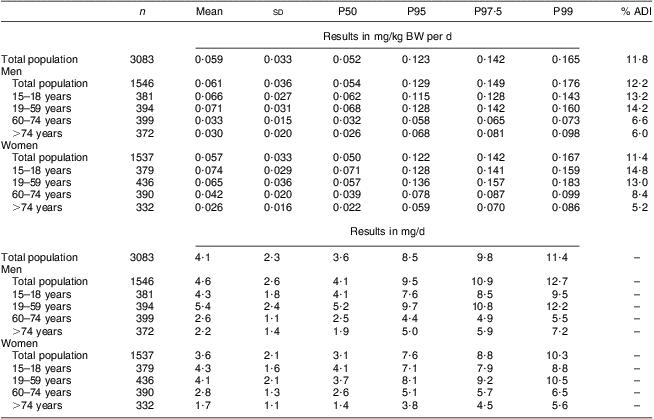
BW, body weight; P, percentile; ADI, acceptable daily intake.
The mean total lycopene intake was 0·059 mg/kg BW per d. At none of the percentiles was the ADI of 0·5 mg/kg BW per d exceeded. Even at the 99th percentile, the total lycopene intake was only 0·165 mg/kg BW per d which is about 33 % of the established ADI value (Table 2 and Fig. 1). The intake of cis-lycopene among the Belgian population was 0·019 (sd 0·011) mg/kg BW per d, which represents about 30 % of the total lycopene intake. Similar to total lycopene, cis-lycopene intake was higher among men than women, although to a slightly lower extent (Table 3).
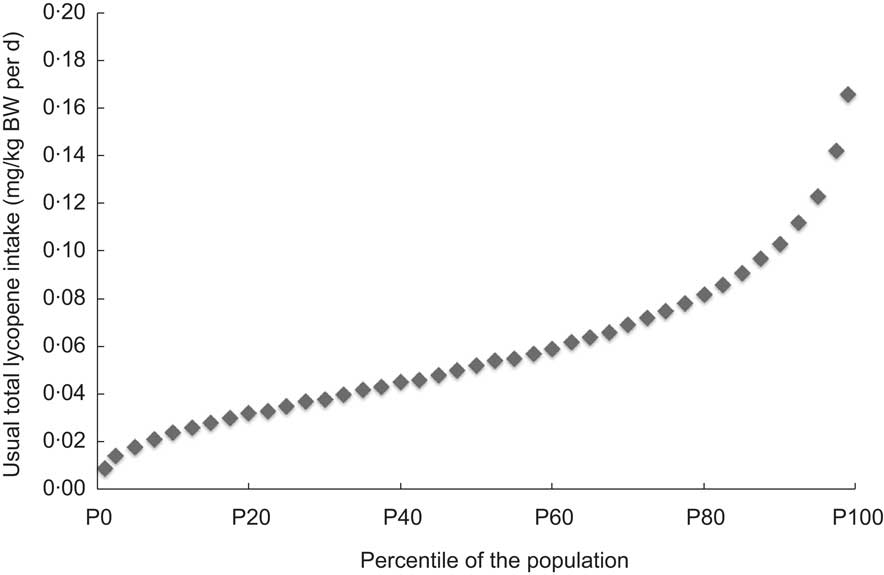
Fig. 1 Estimated usual daily intake of total lycopene from natural sources (mg/kg body weight (BW) per d) of the Belgian adult population (n 3083, all days, all population); Belgian Food Consumption Survey (BFCS), 2004
Table 3 Estimated cis-lycopene intake from natural sources (in mg/kg BW per d and in mg/d) of the Belgian adult population; Belgian Food Consumption Survey (BFCS), 2004
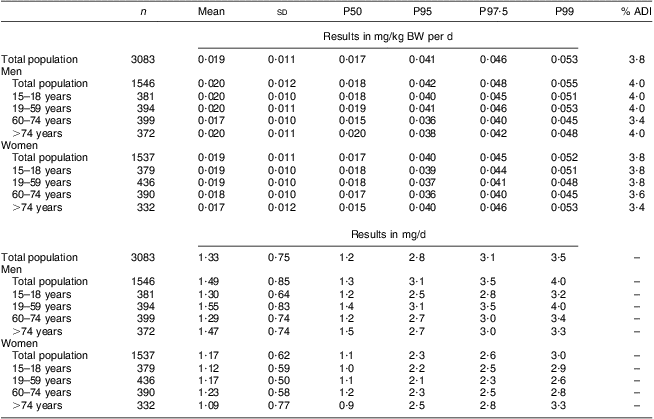
BW, body weight; P, percentile; ADI, acceptable daily intake.
Table 4 shows the food sources contributing to lycopene intake for the Belgian adults. From Table 4 it is obvious that the main contributors to lycopene intake in the Belgian population were the group of tomatoes and tomato products (43 %), together with the group of sauces and ready-to-eat meals (41 %). These two food groups accounted for about 85 % of the total lycopene intake.
Table 4 Contribution of several food groups to the mean intake of total lycopene from natural sources (mg/kg BW per d) of the Belgian population (all population, 6166 interviews, 3083 respondents); Belgian Food Consumption Survey (BFCS), 2004
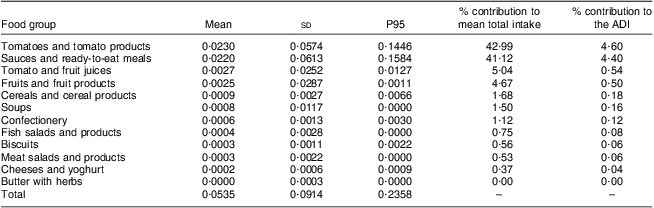
BW, body weight; P, percentile; ADI, acceptable daily intake.
Discussion
The results of the present study show that the mean intake of lycopene among Belgian adults was 0·059 mg/kg BW per d. The lycopene intake of the Belgian population was well below the ADI which was set by the European Food Safety Authority. Intake of cis-lycopene, which may be formed upon processing of foods involving heat and which is more bioavailable than trans-lycopene, represented about one-third of total lycopene intake. When comparing the lycopene intake of the Belgian adult population with that of populations in other countries, it was observed that the intake in Belgium is comparable to the intake of adults in the Netherlands, France, Republic of Ireland and Australia (Table 5)( Reference Porrini and Riso 27 ). Reported lycopene intakes in the USA and Canada were much higher due to the difference in dietary assessment methods used. It was previously shown that mean dietary intakes of lycopene estimated with FFQ (as in the case of intake assessment for the USA and Canada) may be higher than those obtained with diet records( Reference VandenLangenberg, Brady and Nebeling 28 ), both estimated records and weighed records( Reference Yong, Forman and Beecher 29 , Reference Carroll, Corridan and Morrissey 30 ). The high intakes obtained with FFQ may result from a tendency of individuals to overestimate the consumption of vegetables and fruits when presented with a long list of food items. Differences between countries may also be linked to different dietary habits, the quality of the food composition database used and the variation of lycopene concentration within foods.
Table 5 Dietary lycopene intake from natural sources in selected countries( Reference Porrini and Riso 27 )

BW, bodyweight.
*Considering that mean BW is 70 kg.
The current study presents data on lycopene intake of the Belgian adult population. The total and cis-lycopene intakes were calculated based on their determination in a number of food products using an in-house analytical method( Reference Cucu, Huvaere and Van Den Berghe 22 ). Therefore no variability regarding differences in analytical method influenced the results for intake.
Since no food products with added lycopene as a colour were found on the Belgian market, the reported intake represents the intake from natural sources only. The lack of an E160d number of lycopene on food labels is probably due to the fact that industry uses tomato-based powders or paste as addition instead of the additive.
To date, no recommended dietary intake levels have been established for lycopene. However, based on the established ADI value we can imply that no more than 35 mg of lycopene (0·5 mg/kg BW per d × 70 kg average BW) should be ingested on a daily basis. Lycopene may have beneficial effects to health. One meta-analysis reported a protective effect of lycopene on serum cholesterol and blood pressure( Reference Ried and Fakler 31 ). The study on serum lipids revealed a significant cholesterol-lowering effect of lycopene for serum total cholesterol (mean change: −7·55 (se 6·15) mg/dl; P = 0·02) and LDL cholesterol (mean change: −10·35 (se 5·64) mg/dl, P = 0·0003) in the subgroup of trials using lycopene dosages of ≥25 mg daily, whereas subgroup meta-analysis of trials using lower lycopene dosages was not significant. Meta-analysis of the effect of lycopene on systolic blood pressure of all trials suggested a significant blood-pressure-reducing effect (mean systolic blood pressure change: −5·60 (se 5·26) mmHg, P = 0·04). In our study however, lycopene intakes were lower than the 25 mg/d for the whole distribution of intake. On the other hand, increasing evidence suggests that a single serving of tomatoes or tomato products ingested daily may contribute to protection from DNA damage. As DNA damage seems to be involved in the pathogenesis of prostate cancer, the regular ingestion of tomatoes or tomato products might reduce the risk of disease( Reference Ellinger, Ellinger and Stehle 32 ). Another study among 27 261 US women suggested that consuming ≥10 compared with <1·5 servings of tomato-based food products weekly results in clinically modest but significant improvements in total cholesterol, total cholesterol:HDL cholesterol ratio and glycated Hb (HbA1c) but not in other coronary biomarkers( Reference Sesso, Wang and Ridker 33 ).
Limitations of the current study include the fact that products were analysed as purchased instead of as consumed, which may have underestimated especially cis-lycopene intake. Many factors affect lycopene bioavailability and absorption such as food processing, the presence of fat and individual variability in absorption capacity. These were not taken into account in the present study. Another important aspect to consider is that any given food source may vary greatly in lycopene content, because of differences in cultivar, technological processing, domestic cooking, etc. This was also not taken into account. Many more analyses on lycopene concentration in foods would be needed and a probabilistic intake assessment would have to be performed to take into account variation in lycopene concentration within foods.
Conclusions
The results of the present study showed that the mean lycopene intake of the Belgian adult population was about 4·1 mg/d or 0·059 mg/kg BW per d. This intake was comparable to the lycopene intake in neighbouring countries and was well below the ADI value established by the European Food Safety Authority. Tomatoes and tomato products together with ready-to-eat meals containing tomato sauces were the products contributing the most to the lycopene intake.
Acknowledgements
Sources of funding: The authors acknowledge the financial support of the Federal Public Service of Health, Food Chain Safety and Environment. Conflicts of interest: The authors declare that they do not have any conflict of interest with regard to this study. Authors' contributions: S.V. and T.C. wrote the paper and contributed equally. T.C. performed the laboratory analyses and S.V. performed the statistical analyses. All authors contributed to the design of the study and critically revised draft versions of the manuscript.










